 [7]
[7] 

This section addresses the burden of musculoskeletal diseases on specific populations. Included are sex and gender, the aging population, children and adolescents, and differences found among ethnic and racial populations, and the populations of four geographic regions in the US.
Demographic shifts have changed the landscape of the United States. The growth in the number and proportion of older adults is unparalleled in US history. Aging baby boomers and longer life spans combined will double the population of older Americans (age 65 years or older) during the next 25 years to about 72 million. By 2030, older adults will account for roughly 20% of the US population.1
During the past century, there has been a change in the leading causes of death for all age groups, including older adults, from infectious diseases and acute illnesses to chronic diseases and degenerative illnesses. Nearly half (42%) of all Americans, and four of every five older Americans, have numerous chronic conditions.2 Treatment for this chronic-conditions population accounts for 90% of the country’s 3.5 trillion annual healthcare expenditures.2, 3
The ability to move (mobility) is essential to everyday life and central to health and well-being among older populations. Impaired mobility is associated with a variety of unfavorable health outcomes. As the proportion of older Americans continues to increase, aging and public health professionals have a role to play in improving mobility for older adults. Gaps exist in the assessment and measurement of mobility among older adults who live in the community, particularly those who have physical disabilities or cognitive impairments.
Older adults are prone to higher rates of nearly all musculoskeletal conditions than those found in younger people. In large part, these conditions can be attributed to wear and tear on bones and joints over a lifetime. However, some musculoskeletal conditions such as back pain are equally prominent in younger age populations, particularly those in their middle ages.
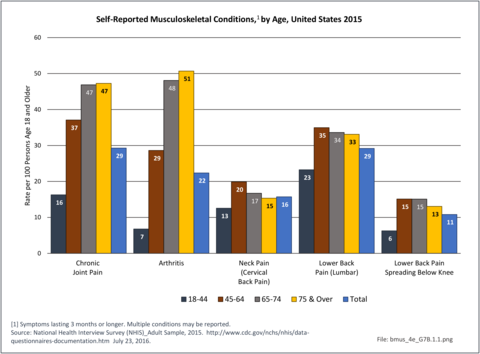
Arthritis is self-reported in 2015 at the highest rate among persons aged 75 years and older (51%), but by nearly as many in the 65 to 74-year age range (48%). Only 29% of persons age 45 to 64 years self-report they have a form of arthritis. Chronic joint pain has a similar reporting pattern as arthritis, but with somewhat lower rates – 47% among those 65 and older and 37% by those 45 to 64-years of age. Low back pain, on the other hand, was self-reported at the highest rate by persons aged 45 to 64-years (35%), closely followed by all people age 65 years and older (34%). Overall, 124.6 million people age 18 years and older self-reported one or more types of musculoskeletal conditions in 2015. (Reference Table 7B.1 PDF [4] CSV [5])
The most common joint reported by the 73 million people over the age of 18 years with chronic pain in 2015 is the knee (47 million), followed by the shoulder (22 million). However, the rate per 100 persons in the various age groups reporting chronic pain in specific joints varies. Knee pain (32%) and hip pain (14%) are reported at the highest level by those age 75 and older, while shoulder (15%), fingers (15%), ankle (8%), wrist (9%), and toes (6%) are reported highest by persons age 65 to 74. The only site with the highest reported chronic pain by persons age 45 to 64 years is the elbow (7%). All age groups reported chronic pain in a mean of just over two joint sites. Overall, joint pain in the ankle, wrist, elbow, and toes is lower among those in the oldest age group compared to those 45 to 74 years of age, possibly due to this population segment being less active and placing lower stress on these joints. (Reference Table 7B.1 PDF [4] CSV [5])
Self-reported limitations in performing activities of daily living from arthritis and back or neck problems affect about one in ten people. Limitations caused by arthritis increase steadily as the populations ages, while back and neck problems are relatively consistent after the age of 45. While overall persons age 18 to 45 reported few limitations, back and neck problems are the most common cause. (Reference Table 7B.1 PDF [4] CSV [5])
Bed and Lost Workdays
People age 45 to 64 years accounted for 41% of the 54 million persons age 18 and over who reported bed days in 2015 due to musculoskeletal conditions, but 50% of the total bed days reported taken. A bed day is defined as one-half or more days in bed due to injury or illness, excluding hospitalization. The greater number of total bed days reported by this age group is due to a high mean of 24.8 days per person combined with large share of the population reporting bed days because of a musculoskeletal condition. (Reference Table 7B.1 PDF [4] CSV [5])
This same age group also accounted for slightly more than half (51%) of the 36 million lost workdays due to musculoskeletal conditions reported by people age 18 years and older and in the workforce. People aged 65 years and older reported only 6% of total lost workdays because of the low number that are still in the workforce. (Reference Table 7B.1 PDF [4] CSV [5])
Older adults will often experience musculoskeletal diseases affecting the spine, with spondyloarthritis and osteoporosis with vertebral fractures often the cause of pain and functional decline. Seniors with such problems may find themselves unable to push or pull large objects, or at times even to reach above their heads. They may have problems lifting grocery bags from the floor or completing household tasks. Bending at the waist may increase the risk for vertebral fractures in people with osteoporosis, reducing breathing capacity and predisposing older adults to chronic lung disease and pneumonias.
Self-reported back and neck pain rates peaked in the age range of 45 to 64 in 2015 and was reported at slightly lower rates for persons age 65 years and older. More than one in three people age 45 years and older reported back or neck pain. (Reference Table 7B.2 PDF [16] CSV [17])
People aged 65 and older had the highest rate of healthcare visits for back and neck pain (43.7 per 100 persons) but accounted for only 26% of the 83 million total healthcare visits for back or neck pain in 2013. The rate of healthcare visits for people age 45 to 64 years was nearly as high (41.0 per 100 persons) and accounted for nearly one-half (48%) of total visits. While only 19.1 in 100 people ages 18 to 44 years had a healthcare visit in 2013 for back and neck pain, this age group comprised 30% of all visits. Total healthcare visits included hospital discharges, emergency department (ED) and outpatient clinic visits, and physician office visits. (Reference Table 7B.2 PDF [16] CSV [17])
Those aged 45 to 64 years had the highest number of spinal fusion procedures performed for back or neck pain, with one in four, or 25.3%, of hospital discharges in this age group with a back or neck pain diagnosis having had a spinal fusion procedure performed. However, the highest rate of hospital discharges with a fusion procedure was among those under 18 years of age (74%), primarily due to the very small number of discharges for back pain in this age group. (Reference Table 7B.2 PDF [16] CSV [17])
Most bed days reported due to back pain (90%) are accounted for by people under the age of 65. A higher number of younger people, those aged 18 to 45 years, report taking bed days than those aged 45 to 64, for spine pain. However, because they report a lower mean number of bed days than the older cohort (6.2 days versus 9.0 days) they account for a slightly smaller share of the total bed days for back pain. People aged 65 years and older account for only a small share of the people who report taking a bed day due to spinal pain (5%), but a larger mean number of days (14.9 days). (Reference Table 9B.2 PDF [16] CSV [17])
Lost workdays due to spine pain or problems in 2015 were taken primarily by people aged 18 to 64 years (96%), the prime workforce ages, and split nearly equally between those under and over the age of 45 years. In 2015, 264 million workdays were reported lost due to back pain. (Reference Table 9B.2 PDF [16] CSV [17])
This report includes a range of deformity conditions that affect the spine. The most common spinal deformity in older adults is acquired through multiple vertebral fractures resulting in kyphosis. Vertebral fractures are often not clinically identified and may show merely as height loss. Nonetheless, vertebral fractures greatly increase the likelihood of future fractures and mortality.1,2
The most familiar spinal deformity condition is that of curvature of the spine, which includes scoliosis, kyphosis, and lordosis. In addition to curvature of the spine, other spinal deformity conditions include spondylolisthesis, spinal infections, complications of surgery, and spondylopathies. Of the 23.4 million healthcare visits in 2013 for spinal deformity, 13 million had a diagnosis of spondylopathy, which refers to any disease of the vertebrae or spinal column associated with compression of peripheral nerve roots and spinal cord, causing pain and stiffness.
Two spinal deformity conditions stand out in the 65 and older cohort -- traumatic spinal fractures and curvature of the spine. People aged 65 years and older accounted for the largest share of healthcare visits in 2013 for vertebral compression fractures (49%), even though they represent only 14% of the population. This group also has a higher than expected share of healthcare visits for all spinal deformity diagnoses (32%). Of the 23.4 million visits in 2013 with a diagnosis of spinal deformity, 40% were made by people age 45 to 64 and 25% by those aged 65 and older. (Reference Table 9B.3 PDF [28] CSV [29])
Arthritis is one of the most common chronic conditions found in the US population. It currently affects 54.4 million adults1 and is projected to reach 78.4 million, or 26% of the adult population by 2040.2 Arthritis is the most common cause of disability in the United States and is a major cause of work and activity limitations, which subsequently affects the economy. Pain from arthritis can substantially affect a person’s quality of life.
Arthritis and other rheumatic conditions (AORC) affect people in higher numbers as they age. Only 7 in 100 persons between the ages of 18 and 44 years report they have doctor-diagnosed arthritis. By the age of 65 years and older, this rate has increased to one in two with some form of arthritis. Although the rates of persons reporting limitations in performing activities of daily living are lower, there is a large disparity between younger persons and the aging. (Reference Table 7B.4.1 PDF [32] CSV [33])
Bed days occur when a person spends at least one-half day in bed in the previous 12 months due to a health condition. On average in the years 2013 to 2015, 607.0 million bed days were reported by persons age 18 years and older due to arthritis. Only 4% of people aged 18 to 44 years reported arthritis-caused bed days. For all people aged 45 years and older, the rate was between 15% and 18%. (Reference Table 7B.4.1 PDF [32] CSV [33])
Arthritis is most likely to be the cause of lost workdays among people between the ages of 45 and 64 years, with nearly 1 in 10 reporting workdays lost. On average in the years 2013 to 2015, 180.9 million workdays were reported lost due to arthritis, with people in the 45- to 64-year age group accounting for 62% of these days. This higher share of lost workdays for this group is likely due to the much higher participation in the workforce for this prime working age cohort. (Reference Table 7B.4.1 PDF [32] CSV [33])
The prevalence of clinically diagnosed symptomatic knee osteoarthritis (OA) was calculated from the National Health Interview Survey 2007–2008 and the proportion with advanced disease (Kellgren-Lawrence grades 3–4) was derived using the Osteoarthritis Policy Model, a validated simulation model of knee osteoarthritis. About 14 million persons have symptomatic knee OA, with advanced OA comprising over half of those cases. This includes more than 3 million African American, Hispanic, and other racial/ethnic minorities. Adults under 45 years of age represented nearly 2 million cases of symptomatic knee OA and individuals between 45 and 65 years of age 6 million more.3
Despite the frequency of severe pain often experienced with arthritis and other rheumatic conditions, these illnesses account for only 21% of the nearly 30 million hospital discharges in 2013. Visits to a physician’s office, emergency department, or outpatient clinic account for most healthcare visits related to arthritis and other rheumatic conditions (AORC), with nearly 100 million ambulatory visits in 2013. Among the 6.4 million hospital discharges for an AORC in 2013, age was a factor in increasing rates of hospitalization. Fewer than 1 in 100 persons ages 18 to 44 years had a hospital discharge with a diagnosis of an AORC, while 9 in 100 aged 65 years and older were discharged with an AORC diagnosis. However, among all AORC conditions, the distribution of healthcare visits by age varied by age group. (Reference Table 7B.4.2 PDF [42] CSV [43])
Osteoarthritis is the primary form of arthritis to affect older persons and begins to show increasing rates for people in their 40s and 50s. Joint pain, the other common problem, results in healthcare visits among people aged 45 to 64. By the age of 65 years, multiple forms of arthritis are often diagnosed and categorized as other rheumatic conditions. (Reference Table 9B.4.2 PDF [42] CSV [43])
Age is not a factor in the length of hospital stay or mean charges with a diagnosis of an AORC. In general, the type of AORC is also not a factor in length of stay or charges. Hospital charges are a rough estimate of hospital cost, and do not include doctor’s fees. (Reference Table 9B.4.3 PDF [46] CSV [47])
Joint replacement procedures are often performed when arthritis has become severe and debilitating. Most procedures are performed on people aged 65 and over, with the exception of spine replacement procedures. (Reference Table 9B.4.4 PDF [48] CSV [49])
Osteoporosis develops when more bone is lost (resorbed) than is replaced in the normal bone remodeling process. Several factors contribute to the development of osteoporosis, but the exact reason why the remodeling process becomes unbalanced is unknown. Factors that often lead to osteoporosis include aging, physical inactivity, reduced levels of estrogen, excessive cortisone or thyroid hormone, smoking, and excessive alcohol intake. Loss of bone calcium accelerates in women after menopause.
Bone loss occurs most frequently in the spine, lower forearm above the wrist, and upper femur or thigh, the site where hip fractures usually occur.
Osteopenia or low bone mass: A value for bone mineral density more than 1 standard deviation (SD) below the young healthy female adult mean, but less than 2.5 SD below this value.1
Osteoporosis: A value for bone mineral density 2.5 SD or more below the young healthy female adult mean.1
Young female adult mean and standard deviation (SD): For the femoral neck, the mean and SD were based on data for 20- to 29-year-old non-Hispanic white females from the Third National Health and Nutrition Examination Survey (NHANES III).2 For the lumbar spine, the mean and SD were based on data for 30-year-old white women from the dual-energy x-ray absorptiometry (DEXA) densitometer manufacturer.3
Other races: People from racial and ethnic groups other than non-Hispanic white, non-Hispanic black, or Mexican American. This group consists primarily of Hispanic descent other than Mexican American, Asian, Native American, and multiracial persons, among others.
Prevalence estimates of osteoporosis or low bone mass at the femoral neck or lumbar spine (adjusted by age, sex, and race/ethnicity to the 2010 Census) for the non-institutionalized population age 50 years and older from the National Health and Nutrition Examination Survey 2005–2010 US Census population counts to determine the total number of older US residents with osteoporosis and low bone mass. There were over 99 million adults 50 years and older in the US in 2010. Based on an overall 10.3% prevalence of osteoporosis, the authors estimated that in 2010, 10.2 million older adults had osteoporosis. The overall low bone mass prevalence was 43.9%, from which they estimated 43.4 million older adults had low bone mass. Of these, 7.7 million were non-Hispanic white (prevalence of 10.2%), 0.5 million non-Hispanic black (prevalence of 4.9%), and 0.6 million Mexican American adults (prevalence of 13.4%) had osteoporosis and another 33.8 million, 2.9 million, and 2.0 million had low bone mass (prevalence 44.9%, 29.7%, and 43.2%), respectively. 4 (Reference Table 7B.5.1 PDF [52] CSV [53])
Osteoporosis often is not the principal diagnosis code related to a healthcare visit because the condition is usually an underlying cause of another condition, particularly fragility fractures that often occur after a fall or other seemingly minor incident. Often in such healthcare visits, osteoporosis may not even be listed as a condition. Still, in 2015, primary osteoporosis was listed in 1.87 million hospital discharges and emergency department visits as a reason for the visit in the population aged 50 and over. Fragility fractures occurred in 1.48 million visits for people aged 50 years and older. (Reference Table 7B.5.1 PDF [52] CSV [53])
Age is a factor in both primary osteoporosis diagnosis and in the occurrence of fragility fractures with most occurring in people after the age of 70. A prior fracture in women aged 50 years and older is the most important risk factor for hip fractures. More than three-fourths (76%) of primary osteoporosis diagnoses were for people ages 70 years and older. However, in 2013, 8% of osteoporosis diagnoses was for people aged 50 to 59 years, and 16% among those aged 60 to 69 years.
Among fragility fractures, 79% were for people aged 70 years and older, with the remainder split among those aged 50 to 69. The site of the fracture was particularly important with respect to age. The oldest group, those 70 years and older, were prone to fractures of the hip and vertebrae. Fractures of the wrist and ankle or foot occurred across all people over the age of 50. (Reference Table 7B.5.1 PDF [52] CSV [53])
Approximately 30% of older women will fall annually, and this risk may be higher in women with other chronic conditions.5,6 Several studies have used survey data to analyze falls, while other studies have limited their analysis to falls seen in emergency departments, in older women, or falls that resulted in fracture or hip fracture.
Falls prevalence may vary by race/ethnicity. In a survey-based cross-sectional study of self- reported falls from 6,277 women 65–90 years of age. The independent association of race/ethnicity and recent falls was examined, adjusting for known risk factors. Compared to whites, Asian (OR 0.64, CI 0.50–0.81) and black (OR 0.73, CI 0.55–0.95) women were much less likely to have ≥1 fall in the past year, adjusting for age, comorbidities, mobility limitation and poor health status. Asians were also less likely to have ≥2 falls (OR 0.62, CI 0.43–0.88). This may contribute to their lower rates of hip fracture.7
Fractures are associated with significant increases in health services utilization compared to pre-fracture levels. Relative to the prior 6-month period, rates of acute hospitalization are between 19.5 (distal radius/ulna) and 72.4 (hip) percentage points higher in the 6 months after fractures. Average acute inpatient days are 1.9 (distal radius/ulna) to 8.7 (hip) higher in the post-fracture period. Fractures are associated with large increases in all forms of post-acute care, including post-acute hospitalizations (13.1% to 71.5%), post-acute inpatient days (6.1% to 31.4%), home healthcare hours (3.4% to 8.4%), and hours of physical (5.2% to 23.6%) and occupational therapy (4.3% to 14.0%). Among patients who were initially community dwelling at the time of the initial fracture, 0.9% to 1.1% were living in a nursing home 6 months after the fracture. These rates rose by 2.4% to 4.0% one year after the fracture.8
Since 1980, there has been a nearly 15% decrease in the prevalence of chronic disability and institutionalization among people aged 65 years and older. A reduction in disability translates directly into cost savings since it is seven times more expensive to care for a disabled senior versus a healthy one. Major activity limitations are a common cause of nursing home admissions. While the most common cause of limitations is arthritis, affecting nearly 50% of people older than 65 years and an estimated 60 million by 2020.9
Vertebral and hip osteoporotic fractures result in a 20% increase in mortality, usually observed in the 12 months after the fracture. Men, who are generally older at the time of the hip fracture, have a 30% mortality rate after the fracture. Moreover, comorbidities such as cardiovascular disease contribute to a higher mortality rate.
A population-based study in Olmsted County, MN, found that within the first seven days after hip fracture repair, 116 (10.4%) of participants experienced myocardial infarction and 41 (3.7%) subclinical myocardial ischemia. Overall, the 1-year mortality was 22%, with no difference between those with subclinical myocardial ischemia and those with no myocardial ischemia. One-year mortality for those with a myocardial infraction was significantly higher (35.8%) than for the other two groups.10 The relative mortality after vertebral fracture varies from 1.2 to 1.9 in different reports,3,11 but the excess deaths occur late, rather than early, after vertebral fractures.12
For older adults, falls and associated injuries threaten health, independence, and quality of life. More than a third of people aged 65 years and older who live independently fall each year; falls are the leading cause of injury-related deaths and hospital emergency department visits.
On average, more than 8.7 million injury episodes, of which 3.1 million were fall related, for which people sought medical treatment were self-reported by individuals in 2013-2015. The majority of injuries occurred to people between the ages of 18 and 64 years, the ages that comprised 83% of the over-18-year population in the United States. Sprains and strains (31%) were the most frequent injury reported for which medical care was sought, but 18% suffered fractures, 18% severe contusions, and 14% open wounds.
Falls are the primary cause of musculoskeletal injuries as the population ages. Approximately three out of four injuries among people aged 65 years and older for which a person is hospitalized or visits an emergency department is the result of a fall. Trauma, such as auto accidents and other accidents involving machinery or moving objects, is a major cause of musculoskeletal injuries among people ages 18 to 44 years, particularly for injuries where care is received in an emergency department. Other causes of injuries, including sports injuries, are seen in emergency departments for one in three (33%) injuries to people aged 18 to 44 years and one in two (51%) for people under the age of 18. (Reference Table 9B.6 PDF [63] CSV [64])
Osteogenic sarcoma (OS) exhibits a bimodal distribution, the significant second peak in incidence occurs in the seventh and eighth decades of life. Osteosarcoma in the elderly can also be attributed to Paget’s disease or previous radiotherapy. The expectation that these elderly patients may not tolerate aggressive modern chemotherapy means that those patients who develop OS after the age of 40 years are excluded from current trials of treatment. As a result, remarkably little is known about the outcome for this age group.1
The overall incidence of tumors of the musculoskeletal system is lower than many types of cancers. This is particularly true for primary cancers of the bones and joints, although bones and joints are frequently a site of secondary, or metastasized, cancers. The occurrence of cancers of the bones and joints affects all ages and is one of the primary cancers in young people. Myeloma, cancer of the bone marrow, is a disease of the elderly, with nearly two-thirds of cases found in persons age 65 and over. Soft tissue cancers affect all ages, but the occurrence increases with age. (Reference Table7B.7 PDF [67] CSV [68])
Key challenges in the area of musculoskeletal health in older adults are significant. Along with the dramatic increase in the number of older adults is the expectancy that healthy adults will maintain mobility and activity. However, prolonged life expectancy and years of stress on bodies is greatly increasing the likelihood of development of arthritis and osteoporosis, among other conditions, over the years. These conditions often lead to pain, disability, and reduce the ability to remain active and perform activities of daily living. New research to address causes and reduce disability caused by conditions common in the aging population is needed.
A growing body of work on health-related knowledge translation1 reveals significant gaps between what is known to improve health, and what is done to improve health.
A gap in medical care continues to occur after an osteoporosis related fracture in older adults. Furthermore, the decreasing rate of treatment for osteoporosis after hip fracture is noted in the US by Solomon and colleagues.2 The latest quality measures by the National Commission on Quality Assessment (2017) indicate that treatment for osteoporosis after a fracture in an older woman has increased. Evaluation measured as a bone density test is performed in 72.7% of health management organizations (HMOs) and 82% of Preferred Provider Organizatons (PPO). Actual osteoporosis treatment is reported as 46.7% of HMOs and 39.1% of PPOs. This is a substantial increase over prior annual findings.
System-based quality improvement programs such as the American Orthopaedic Association’s “Own the Bone [71]” have been successful with raising awareness and spearheading improvement in increasing treatment rates for osteoporosis after a fracture.3,4,5
Another area with a gap in medical care is in the prevention in falls. Falls are common in older individuals, affecting as many as 30% of older women. Injuries from falls include fractures and blunt head trauma, and result in increased mortality. Women with self-reported osteoarthritis (OA), in particular, have an increased risk of falls, and in spite of elevated bone mass, remain at risk of fractures.6 In 2017, the cost of fall injuries totaled as much as $49.5 billion, depending on methods used to identify a fall, the national healthcare database used, and study design.7 As the population ages, the financial toll for older adult falls is projected to reach $67.7 billion by 2020.8
Falls result in more than 2.8 million injuries treated in emergency departments annually, including over 800,000 hospitalizations and more than 21,000 deaths. Every 11 seconds, an older adult is treated in the emergency room for a fall. Every 19 minutes, an older adult dies after a fall.8
In conclusion, musculoskeletal disorders are prevalent, and often of serious consequences in older adults. A greater awareness in osteoporosis care after a fracture can be helped through bone density testing. The use of osteoporosis therapy afater fracture will result in a higher quality of life and prevention of disability among America’s seniors.
Previous sections in this text clearly demonstrate the large percentage of healthcare visits that are attributable to musculoskeletal conditions. Most of the data used to establish these estimates concern adult patients. Unfortunately, there is significantly less information regarding the burden of these conditions in young patients.
Studies, however, do support that pediatric musculoskeletal conditions similarly account for a significant portion of visits to medical providers. For instance, de Inocencio reported that greater than 6% of total visits to pediatric clinics were for musculoskeletal pain.1 Schwend reported that approximately one third of pediatric medical problems are related to the musculoskeletal system.2 In a population-based study in Ontario, Canada, Gunz reported that 1 in 10 children made a healthcare visit for a musculoskeletal problem and that 13.5% of all visits for musculoskeletal disease were made by patient’s age 0 to 19 years.3 Four in 1,000 children are reported by parents as having difficulty with activities of daily living due to musculoskeletal conditions. A search of the National Health Interview Survey [73] (NHIS) child sample revealed that musculoskeletal conditions accounted for 1.6% of parent-reported health conditions in 73.5 million healthcare visits for children and adolescents age 0 to 17 years in the US from 2013 to 2015. This proportion was greatest at 2.4% in the 14- to 17-year-old age group. (Reference Table 7C.0 PDF [74] CSV [75])
The evaluation and treatment of these pediatric musculoskeletal conditions resulted in approximately 94.8 million missed school days per year from 2013 to 2015, accounting for 27.5% of all missed school days. Musculoskeletal conditions are surpassed only by respiratory infections and developmental delay as a cause of missed school days. Children aged 5 to 9 years old missed the highest number of school days due to musculoskeletal pain. (Reference Table 7C.0.1 PDF [76] CSV [77])
Children with musculoskeletal conditions also commonly have other medical problems. According to the National Health Interview Survey from 2013 to 2015, these are most commonly respiratory conditions followed by developmental delay. Of children with musculoskeletal conditions, 48% also have a diagnosed respiratory condition and 36% have developmental delay. (Reference Table 7C.0.2 PDF [80] CSV [81])
Despite the significant contribution made by musculoskeletal conditions in the total US healthcare burden, research for pediatric musculoskeletal conditions is grossly underfunded. Of the $3.25 billion in National Institutes of Health (NIH) research funding for all pediatric conditions in 2013, only $46.8 million, or 1.4% of total pediatric medical research funding, went toward pediatric musculoskeletal research. Even under the umbrella of funding specifically for musculoskeletal research, pediatric-specific research is under-represented. Of the $424.4 million in funding for the National Institute of Arthritis and Musculoskeletal and Skin Disease (NIAMS) in 2013, this same $46.8 million represented only 11% of total musculoskeletal research dollars.4
In order to perform a comprehensive review of the burden of musculoskeletal disease in children and adolescents, all conditions that are direct musculoskeletal diagnoses or have musculoskeletal implications were considered for this section. This chapter was divided into separate clinically relevant sections to better understand the burden of each. These sections include musculoskeletal infections, deformity, trauma, neuromuscular conditions, syndromes with musculoskeletal implications, sports injuries, neoplasms, skeletal dysplasias, rheumatologic conditions, medical problems with musculoskeletal implications, and pain syndromes.
Healthcare visits and hospitalization data are derived from diagnostic codes for each of the conditions presented. These codes are available in the ICD-9-CM Codes [84] section of this topic. Total healthcare visits are the sum of cases seen in physicians’ offices (National Ambulatory Medical Care Survey), outpatient clinics (National Hospital Ambulatory Medical Care Survey), emergency departments (Nationwide Emergency Department Sample), and hospital discharges (Nationwide Inpatient Sample). The largest database used is the Healthcare Cost and Utilization Project [85] (HCUP) Nationwide Emergency Department Sample (NEDS), which estimates approximately 32.5 million weighted visits of children and adolescents through the age of 20 years. These four databases were analyzed for the ages 0 through 20 years, with subsets of data by age groups under 1 year, ages 1 to 4 years, 5 to 9 years, 10 to 13 years, 14 to 17 years, and 18 to 20 years.
Each database includes multiple variables to define diagnoses, ranging from three possible diagnoses in the physicians’ office and outpatient clinic data sets to 25 possible diagnoses in the HCUP National Inpatient Sample (NIS) database. If a diagnosis code is listed in any of the possible diagnosis variables, the record is coded as presenting with that condition. If the diagnosis code is listed in the first diagnosis variable, it is coded as the primary diagnosis. However, the databases do not permit diagnostic verification. The first diagnosis listed may not be the primary reason for the visit, but a contributing cause. Further, there is the potential for overlap in diagnosis of related conditions. It is also possible diagnoses codes used for reimbursement purposes may be inaccurate. Therefore, these numbers provide only a guide to the impact of major childhood musculoskeletal conditions.
Injuries include two categories: sports injuries and injuries due to a traumatic event. Sports injuries are identified by type of sports activity using the United States Consumer Product Safety Commission’s National Electronic Injury Surveillance System [88] (NEISS), with annual injuries averaged across the years of 2014 to 2016. Because sports injuries cases are not analyzed by ICD-9-CM codes, they may duplicate trauma injury cases cited from the previously discussed databases.
The 11 categories of musculoskeletal conditions that follow represent the most common healthcare reasons for which children and adolescents are seen in doctors' offices, emergency departments, and hospitals. Many of these conditions, such as the skeletal dysplasias, are relatively rare, diagnosed infrequently in the healthcare system, and have little data available on prevalence and burden. Though rare, they may result in significant morbidity and often require lifelong medical interventions and, therefore, warrant discussion.
In 2013, more than 18 million children and adolescents age 20 years and younger received treatment in medical centers, physicians’ office, and hospitals for a condition that included a musculoskeletal-related condition. More than 65% were for the treatment of traumatic injuries. The second most common diagnosis is a pain syndrome, accounting for more than 1 in 10 visits (15%). Pain syndromes include amplified musculoskeletal pain and benign limb pains, along with less common juvenile primary fibromyalgia syndrome, reflex sympathetic dystrophy, and benign hypermobility syndrome. The third most frequent diagnosis is sports injuries, accounting for just over 10% of all visits. The discussion of sports injuries utilizes a unique database that is not based on ICD-9-CM codes; it is likely there is overlap between traumatic injuries and sports injuries. (Reference Table 7C.1.1 PDF [90] CSV [91])
More than two-thirds (70%) of visits by children and adolescents for a condition that included a musculoskeletal-related condition were to physicians’ offices or outpatient clinics. Hospital discharges accounted for less than 3% of total visits. Healthcare visits that included a musculoskeletal-related condition represented 7% of visits made by children and adolescents for any reason but were more than 15% of all visits to the emergency department. (Reference Table 7C.1.1 PDF [90] CSV [91])
Among the 246 million healthcare visits by children and adolescents in 2013, 14.4 million had a primary diagnosis of a musculoskeletal-related condition. The greater proportion (64%) were for the treatment of traumatic injuries, with the second and third most common primary diagnoses being sports injuries (13%) and pain syndrome (12%). Although all other musculoskeletal-related conditions accounted for 13% of total healthcare visits for a musculoskeletal-related condition, they nevertheless remain serious health concerns for children and adolescents. (Reference Table 7C.1.2 PDF [96] CSV [97])
Again, many visits were to physicians’ offices and outpatient clinics (70%), while visits to an emergency department with a primary musculoskeletal-related condition diagnosis accounted for 29% of visits. Hospital discharges accounted for less than 1% of total visits with a primary musculoskeletal diagnosis. Healthcare visits that included a primary diagnosis of a musculoskeletal-related condition represented 6% of visits made by children and adolescents for any reason but were 13% of all visits to the emergency department. (Reference Table 7C.1.2 PDF [96] CSV [97])
Musculoskeletal infections included in this section are osteomyelitis, septic arthritis, soft tissue infections (myositis), Lyme disease, and tuberculosis. Osteomyelitis and septic arthritis are the most common form of pediatric musculoskeletal infections, and most often occur in the first decade of life in previously healthy children. Infectious myositis refers to conditions causing inflammation in muscles and may be part of a systemic (whole body) infection, especially a viral infection. Lyme disease is caused by a bite from a deer tick and is less common than osteomyelitis and septic arthritis. It is more prevalent in the Northeastern and Midwestern regions of the United States.1 Tuberculosis (TB) has become much less common in the United States over the last few decades but has increased in incidence in developing countries secondary to immunodeficiency and multidrug resistance. TB infections involve the musculoskeletal system in 2% to 5% of cases.2
Community-acquired Staphylococcus aureus (CA-SA) is the most common infecting organism in pediatric musculoskeletal infections and is typically treated with a first-generation cephalosporin, such as cefazolin. Over the past decade, methicillin-resistant Staphylococcus aureus (MRSA) has become prevalent and requires treatment with second-line antibiotics such as clindamycin or vancomycin.3 As MRSA infections have become more prevalent, the disease course for patients with these infections have become much more severe, with greater systemic disease requiring multimodal and multidisciplinary treatments including medical, surgical, and critical care. Patients are often hospitalized for extended periods and most require continued care with long-term antibiotic treatment after discharge. Multiple surgical debridements are often required. Complications of musculoskeletal infections include growth deformity, fractures, and arthritis, and may result in long-term morbidity and dysfunction.
Musculoskeletal infections were diagnosed in 61,400 children and adolescent healthcare visits in 2013, of which 41,800 had a primary diagnosis of musculoskeletal infection. Of this total, 14,000 children and adolescents were hospital discharges, with 8,300 hospitalizations for a primary diagnosis of a musculoskeletal infection. (Reference Table 7C.1.1 PDF [90] CSV [91] and Table 7C.1.2 PDF [96] CSV [97])
Males were more likely to be hospitalized with a musculoskeletal infection than females. The most common age group was between 5 and 9 years old. Musculoskeletal infections as a primary diagnosis accounted for 1.6% of hospital discharges for any musculoskeletal-related condition, but only 0.1% of hospital discharges for all healthcare reasons for children and adolescents age 20 years and younger. (Reference Table 7C.2 PDF [102] CSV [103])
Total charges averaged $76,900 for a mean 8.5-day stay when children and adolescents were hospitalized with a diagnosis of musculoskeletal infection along with other medical conditions. With a primary diagnosis of infection, the stay was shorter (5.9 days), and mean charges were less at $48,300. Total hospital charges for all primary musculoskeletal infection discharges in 2013 were $400.9 million. (Reference Table 7C.2 PDF [102] CSV [103])
Deformity in children and adolescents is subdivided into five sections: upper extremity, lower extremity, hip and pelvis, spine, and other/unspecified.
Upper extremity deformity includes diagnoses such as polydactyly, syndactyly, and reduction deformities such as amyelia and longitudinal deficiencies of the upper extremity, and other congenital deformities such as synostosis, Madelung deformity, and Apert syndrome. A complete listing of deformity codes can be found in the ICD-9-CM Child and Adolescents Codes [84].
Lower extremity deformity includes diagnoses such as polydactyly, syndactyly, and reduction deformities such as amyelia and longitudinal deficiencies of the lower extremity, genu varum, genu valgum, and other congenital developmental deformities such as clubfoot and flatfoot.
Hip and pelvis deformity include diagnoses such as coxa valga, coxa vara, slipped capital femoral epiphysis, pelvic deformity, Legg Calves Perthes disease, and developmental dysplasia of the hip. Hip deformity is among the most common developmental deformities in childhood. Developmental dysplasia of the hip is estimated to occur in between 1 in 100 to 1 in 1000 newborns.1
Spine deformity includes anomalies of the spinal cord such as syringomyelia and diastomatomyelia, as well as deformities of the vertebral column such as scoliosis, kyphosis, spondylolysis, spondylolisthesis, and congenital spinal anomalies.
Other and unspecified deformities include deformities of the chest wall such as pectus excavatum and pectus carinatum, as well as nonspecific deformity diagnoses.
Deformity of the spine represented the largest share of hospitalizations (40.7%) in 2013, followed by the lower extremity at 29% and upper extremity at 19.1%. (Reference Table 7C.3 PDF [108] CSV [109])
Musculoskeletal deformities were diagnosed in 1.7 million children and adolescent healthcare visits in 2013, of which 958,900 had a primary diagnosis of musculoskeletal deformity. Among the total with any diagnoses of deformity, 108,100 children and adolescents were hospital discharges, with 27,500 hospitalizations for a primary diagnosis of a musculoskeletal deformity. (Reference Table 7C.1.1 PDF [90] CSV [91] and Table 7C.1.2 PDF [96] CSV [97])
Females had a slightly higher rate of overall deformity diagnoses with hospitalization, accounting for 52% of primary diagnosis. Neonates had a high rate of musculoskeletal deformity for any diagnosis with hospitalization (24.4%) but accounted for only 0.1% of primary hospitalizations of all musculoskeletal diagnoses. Primary diagnosis of musculoskeletal deformity with hospitalization was highest between the ages of 10 and 17 years.
Musculoskeletal deformity as a primary diagnosis accounted for 5.5% of hospitalizations for any musculoskeletal condition diagnosis, but only 0.4% of hospitalizations for any healthcare reason for children and adolescents age 20 years and under. (Reference Table 7C.3 PDF [108] CSV [109])
Total charges averaged $70,700 for a mean 6.3-day stay when children and adolescents were hospitalized with a diagnosis of musculoskeletal deformity along with other medical conditions. With a primary diagnosis of deformity, the stay was shorter (4.1 days), but mean charges were much higher at $100,200, primarily due to the higher charges for children and adolescents age 10 years and older. Total hospital charges for all primary musculoskeletal deformity discharges in 2013 were $2.76 billion. (Reference Table 7C.3 PDF [108] CSV [109])
Traumatic injury is the leading cause of death in children and adolescents, accounting for 20,000 deaths per year in the United States.1 Although most musculoskeletal injuries are not life threatening, they do account for approximately 10% to 25% of injuries in this age group.2
The pediatric musculoskeletal system is different from that of an adult, and, therefore, the assessment, treatment, and outcome of injuries is different. Pediatric bone is more elastic, and with a capacity for growth, there exists superior remodeling capability. Because of this, many fractures that require surgical treatment in adults may be treated nonoperatively in children. On the other hand, injury to the growing child can result in growth deformity that can lead to long-term morbidity and the need for reconstructive treatments. This section subdivides pediatric musculoskeletal trauma into six sections: upper extremity, lower extremity, hip and pelvis, spine and trunk, birth trauma, and nonaccidental trauma (child abuse). (Reference Table 7C.4 PDF [117] CSV [118])
Trauma resulting in musculoskeletal injury was diagnosed in 11.8 million children and adolescent healthcare visits in 2013, of which 79% (9.3 million) had a primary diagnosis of musculoskeletal injury. Only a small number were serious enough to require hospitalization. Among any trauma musculoskeletal injury diagnoses, 215,200 children and adolescents were hospitalized, with 65,600 having a primary diagnosis of a musculoskeletal injury. (Reference Table 7C.1.1 PDF [90] CSV [91] and Table 7C.1.2 PDF [96] CSV [97])
Males had higher injury rates with hospitalization than females for both any diagnoses (60% of injuries) and as a primary diagnosis (67% of injuries). Hospitalization for musculoskeletal injuries were highest among adolescents age 14 years and older. Neonates under the age of one year had a high rate of musculoskeletal injury for any diagnosis with hospitalization, primarily due to a diagnosis of birth trauma (99%), but a much lower rate of hospitalization with a primary trauma diagnosis (0.5% of musculoskeletal diagnoses in this age bracket).
Musculoskeletal injury as a primary diagnosis accounted for 13% of hospitalizations for any musculoskeletal condition diagnosis, and 1.0% of hospitalizations for any healthcare reasons for children and adolescents age 20 years and younger. For all but the youngest age, which is skewed by birth trauma, primary diagnosis of trauma accounted for 13.5% to 21.9% of all hospitalization for any musculoskeletal diagnoses. (Reference Table 7C.4 PDF [117] CSV [118])
Trauma to the upper extremity account for half (50%) of all trauma healthcare visits by children and adolescents. This was followed by lower extremity trauma (38%). Spine and trunk injuries were 8%, with hip and pelvis injuries at 2%. A diagnosis of birth trauma was less than 1% of all healthcare visits but accounted for more than half (53%) of hospital discharges for musculoskeletal trauma diagnoses. Child abuse was reported in 1% of all healthcare visits for trauma. (Reference Table 7C.1.1 PDF [90] CSV [91])
Total charges averaged $37,100 for a mean 4.2-day stay when children and adolescents were hospitalized with a diagnosis of musculoskeletal injury along with other medical conditions. With a primary diagnosis of musculoskeletal injury, the stay was shorter (3.1 days), but mean charges were higher at $46,300, likely due to the high number of birth trauma cases. Mean charges were highest for older adolescents (18 to 20 years) followed by neonates. Total hospital charges for all primary musculoskeletal injury discharges in 2013 were $3.04 billion. (Reference Table 7C.4 PDF [117] CSV [118])
Common pediatric neuromuscular conditions include cerebral palsy, myelomeningocele (spina bifida), muscular dystrophy, spinal muscular atrophy, hereditary motor sensory neuropathies, Friedrich ataxia, and Rett syndrome. This is a heterogeneous group of disorders with varying degrees of severity and involvement. Although some children and adolescents with these diagnoses can lead a relatively normal life and participate in normal activities, many are completely dependent on their care provider. Most patients lie somewhere between the two ends of this range and require varying amounts of care for their condition. The overall burden of these diagnoses is not limited to number of visits or admissions. These diagnoses also carry significant indirect costs including, but certainly not limited to, lost wages by the caregiver who is unable to go to work; out-of-pocket costs for necessities such as therapy, bracing, and wheelchairs; and the significant emotional impact on the family and care provider.
Neuromuscular conditions were diagnosed in 554,500 children and adolescent healthcare visits in 2013, of which 214,600 had a primary diagnosis of a neuromuscular condition. About 1 in 10 (11%) children and adolescents with any neuromuscular diagnoses were hospitalized (61,200), but fewer than 2% (4,100) with a primary neuromuscular diagnosis had a hospital discharge. (Reference Table 7C.1.1 PDF [90] CSV [91] and Table 7C.1.2 PDF [96] CSV [97])
Males were slightly more likely to be hospitalized than females for both any neuromuscular diagnoses and as a primary diagnosis. Children ages 6 to 10 years had the highest rate of hospitalization, both with any diagnoses and as a primary diagnosis. Rates of hospitalization declined past 9 years old.
Neuromuscular conditions as a primary diagnosis accounted for 0.8% of hospitalizations for any musculoskeletal condition diagnosis and only 0.1% of all hospitalizations for any healthcare condition. (Reference Table 7C.5 PDF [127] CSV [128])
Cerebral palsy was diagnosed in two-thirds (65%) of hospital discharges. Spina bifida and muscular dystrophy represented 18% and 7% of discharges, respectively.
Total charges averaged $75,700 for a mean 6.7-day stay when children and adolescents were hospitalized with a diagnosis of a neuromuscular condition along with other medical conditions. With a primary neuromuscular diagnosis, the stay was longer (7.2 days), and mean charges were higher at $92,000. Mean charges and length of stay were highest for the youngest patients, neonates. Total hospital charges for all primary neuromuscular discharges in 2013 were $377.2 million. (Reference Table 7C.5 PDF [127] CSV [128])
Syndromes with musculoskeletal implications include those diagnoses that may result in or be associated with musculoskeletal problems or deformities. The most common syndromes with musculoskeletal implications include Marfan syndrome, Ehlers Danlos syndrome, Down syndrome, and neurofibromatosis. These patients may have musculoskeletal problems including scoliosis, pectus deformities, hip dysplasia, and flatfeet. Patients with neurofibromatosis may have congenital pseudarthrosis of the tibia. Many of these patients will require treatment for these musculoskeletal problems. Treatment, however, must be tailored to each individual patient as these syndromes often affect multiple body systems and require involvement of multiple medical disciplines.
Syndromes with musculoskeletal implications were diagnosed in 383,200 children and adolescent healthcare visits in 2013, of which 126,300 had a primary diagnosis of one of these conditions. About 1 in 10 (9%) children and adolescents with any syndrome with musculoskeletal implications diagnoses were hospitalized (29,800), but less than 1.2% (600) with a primary diagnosis of a syndrome with musculoskeletal implications had a hospital discharge. (Reference Table 7C.1.1 PDF [90] CSV [91] and Table 7C.1.2 PDF [96] CSV [97])
Male were more likely than females to have a hospital discharge with any syndrome with musculoskeletal implications diagnoses as well as a primary diagnosis. Infants and young children under the age of 5 years had the highest rate of hospitalization for any diagnoses of syndromes with musculoskeletal implications. The number of hospitalizations with a primary diagnosis was too small for analysis by age.
Any diagnoses of syndromes with musculoskeletal implications accounted for 5.4% of hospitalizations for any musculoskeletal condition diagnosis, and 0.4% of all hospitalizations for any healthcare condition. Hospitalizations with a primary diagnosis were 0.1% of all musculoskeletal diagnoses. (Reference Table 7C.6 PDF [135] CSV [136])
Total charges averaged $78,500 for a mean 7.9-day stay when children and adolescents were hospitalized with a diagnosis of a syndrome with musculoskeletal implications condition along with other medical conditions. The number of hospitalizations with a primary diagnosis of a syndrome with musculoskeletal implications was too small for analysis of hospital charges. (Reference Table 7C.6 PDF [135] CSV [136])
Athletic participation by children and adolescents increased dramatically between 1997 and 2008,1 with participation declining slightly since the 2008 peak.2 Over the past several years, participation in some sporting activity has slowly increased with 69% of children playing a sport at least one day during the year in 2017. However, team sports participation regularly continues to slowly decline with only 37% of children consistently participating in a team sport.3
Since the late 1990s, athletic specialization has increased, resulting in earlier focus on single sports. As a result, there has been a commensurate increase in pediatric sports-related injuries, both acute and related to chronic overuse.4 Pediatric and adolescent athletes are anatomically and physiologically different from adult athletes, and therefore are at risk to sustain different injuries. Coordination and mechanics are less developed in pediatric athletes, placing them at greater risk for injuries related to falls and collisions. Growing athletes are at risk for most of the same injuries as adult athletes but are uniquely susceptible to injuries about the physeal (growth plates in bones that undergo endochondral ossification) and growth cartilage. Not only do these physeal and apophyseal injuries5 require unique treatments, but they may also result in growth derangement that can lead to deformity and have long-term consequences. Adolescent female athletes also have been shown to have a two- to nine-fold greater risk of knee injuries, which may be related to age and gender-specific differences in anatomy, neuromuscular control, and hormone levels.6 Common pediatric sports-related injuries include anterior cruciate ligament (ACL) and meniscal tears, tibial eminence fractures, osteochondritis desiccans lesions, patellofemoral instability, Osgood Schlatter syndrome, little league shoulder and elbow, pelvic avulsion fractures, and distal radius epiphysitis.
On average across the years from 2014 to 2016, 1.6 million injuries per year related to team or individual sport activities occurred to children and adolescents age 20 years and younger. Data reported is from consumer product-related injuries occurring in the United States from a statistically valid sample of emergency departments collected by the United States Consumer Product Safety Commission, National Electronic Injury Surveillance System. Data shown for sports injuries are not included in the overall total for musculoskeletal conditions among children and adolescents, on the assumption it duplicates numbers found in the emergency department database based on ICD-9-CM codes and used in the trauma injuries section.
Males report injuries at twice the rate as females (64% of injuries), with the highest number of injuries occurring in the junior high (10 to 13 years) and high school (14 to 17 years) ages. (Reference Table 7C.7.1 PDF [141] CSV [142])
Team sports, both organized and informal, accounted for just under one-half (46%, or 740,200 injuries) of all sports-related injuries reported. Basketball had the highest number of team sport related injuries at 33% and was closely followed by football at 31%.
Team sport injuries to males were three times the number reported for females (75%). The only sport in which female injuries outnumber male injuries is volleyball. Nearly half (45%) of team sport injuries to children and adolescents occurred during the high school years (age 14 to 17 years), with another 34% in the junior-high age range of 10 to 13 years. (Reference table 7C.7.1 PDF [141] CSV [142])
The most common musculoskeletal injury incurred was a sprain or strain, accounting for 47% of team sport injuries. Volleyball had the highest proportion of sprains and strains, followed by basketball. Baseball led in contusion injuries, while fractures occurred most frequently in football, followed by soccer and hockey (including field, ice, and roller hockey). Only 1% of team sport injuries were serious enough to result in hospitalization. (Reference table 7C.7.2 PDF [145] CSV [146])
Individual sports injuries accounted for 54% of total injuries reported (872,900). Almost one in five injuries (18%) occurred while riding bicycles or other nonmotorized wheeled equipment such as tricycles and scooters. These injuries occurred most frequently to children ages 10 to 13 years. Injuries on playground equipment were the second highest type of individual sport injuries, accounting for 15% of all injuries. Playground equipment injuries occurred almost exclusively to children younger than 14 years old and most commonly in children aged 5 to 9 years old. Skating injuries (which includes roller and ice skates, inline skates, and skateboards) were the cause of 11% of individual sport injuries.
Females accounted for a larger share of individual sport injuries (45%) than in team sports. Still, the only activities in which females had a significantly higher number of injuries than males were in gymnastics/cheerleading/dancing as well as track and field. (Reference Table 7C.7.1 PDF [141] CSV [142])
Fractures and sprains/strains each accounted for one-third of all individual sport activity injuries (36% and 36% respectively). However, the type of musculoskeletal injury varied substantially with the type of activity. Fractures resulted from playground equipment injuries more than one-half the time (57%), with a high share of fractures in snow sports (44%) and skating injuries (42%) as well. Sprains/strains occurred in almost two-thirds of track and field injuries (62%), and there were a higher share of sprains/strains occurring in fitness training (59%) and gymnastics/cheerleading/dancing (57%) as well. The most common type of injury reported from bicycle/wheeled equipment was contusions (44%). Nearly 3% of individual sport injuries resulted in hospitalization. (Reference table 7C.7.2 PDF [145] CSV [146])
Pediatric musculoskeletal neoplasms are relatively rare. They can be categorized as either benign or malignant, as has been done for this document. Musculoskeletal neoplasms are often also categorized by the type of tissue they produce or from which they are derived.
The most common types of tumors that affect the musculoskeletal system are cysts, bone-producing tumors, cartilage tumors, fibrous tumors, soft tissue tumors, and peripheral neuroectodermal tumors. Most benign tumors, such as nonossifying fibromas, result in little or no disability and require no treatment. Other benign tumors may require surgical intervention. Painful or prominent osteochondromas may require surgical excision. Simple bone cysts can weaken the bone, increase fracture risk, and may require surgical treatment in order to resolve the cyst and prevent or treat fracture. Other benign tumors include lipomas, fibrous dysplasia, enchondromas, osteoid osteoma, and osteoblastomas.
The most common malignant tumors of the pediatric musculoskeletal system are osteosarcoma, Ewing sarcoma/peripheral neuroectodermal tumor, rhabdomyosarcoma, and synovial cell sarcoma. Osteosarcoma is the most common malignant bone tumor in patients under 20 years of age, with an incidence of approximately 29 per 1 million people. Ewing sarcoma is the second most common pediatric malignant musculoskeletal tumor and is part of the Ewing family of tumors, which includes peripheral neuroectodermal tumors. Most of the tumors in the family have a genetic translocation.1 Long-term survival of patients with both tumors has drastically improved with the routine use of chemotherapy.
For additional information on musculoskeletal tumors in children, you can refer to the Tumors [156] section of this report.
Neoplasms, including both benign and malignant, were diagnosed in 155,500 children and adolescent healthcare visits in 2013, of which 43,100 had a primary diagnosis of a neoplasm. About one in seven (5%) of children and adolescents with any neoplasm diagnoses were hospitalized (23,000), but fewer than 1% of hospital discharges had a primary diagnosis of neoplasm (3,600). (Reference Table 7C.1.1 PDF [90] CSV [91] and Table 7C.1.2 PDF [96] CSV [97])
Slightly more males than females had a hospital discharge with any or a primary neoplasm diagnosis. For each year from birth until 18 years, there is an increasing incidence of neoplasm prevalence resulting in hospitalization.
Any diagnoses of neoplasm accounted for 4.4% of hospitalizations for any musculoskeletal condition diagnosis, and 0.4% of all hospitalizations for any healthcare condition. Hospitalizations with a primary diagnosis of neoplasm accounted for 0.7% of all musculoskeletal diagnoses and only 0.1% of hospitalizations for any health condition diagnosis. Benign neoplasms accounted for 59% of neoplasm diagnoses, but 87% of hospitalized diagnoses are malignant. (Reference Table 7C.8 PDF [159] CSV [160])
Total charges averaged $48,500 for a mean 4.6-day stay when children and adolescents were hospitalized with any diagnosis of neoplasm along with other medical conditions. With a primary neoplasm diagnosis, the stay was slightly longer (6.4 days), and mean charges were higher at $93,100. Mean length of stay was highest for children less than one year of age; however, hospital charges were highest for children ages 5 to 9 years old. Total hospital charges for primary neoplasm diagnosis discharges in 2013 were $335.2 million. (Reference Table 7C.8 PDF [159] CSV [160])
Skeletal dysplasias, also referred to as osteochondrodysplasias, are a heterogeneous group of disorders that affect the growth and development of bone and cartilage. There is great variability of severity and involvement ranging from neonatal lethality to mild growth differences noted incidentally in adulthood. Hundreds of such dysplasias have been described, but most are so rare that true incidence is difficult to estimate.1 The most common diagnoses included in this category are chondrodysplasia, achondroplasia, hypochondroplasia, dwarfism, congenital absence of rib, osteogenesis imperfecta, osteopetrosis, as well as other dysplasias. The overall incidence of skeletal dysplasias is two to five per 10,000 live births.2 Despite their relative rarity, many patients with these disorders require extensive medical and surgical treatments throughout their childhood and into adulthood.
Skeletal dysplasias were diagnosed in 235,800 children and adolescent healthcare visits in 2013, accounting for the primary diagnosis in 47,500 of these visits. (Reference Table 7C.1.1 PDF [90] CSV [91] and Table 7C.1.2 PDF [96] CSV [97])
Males were slightly more likely to be hospitalized for both any musculoskeletal diagnosis as well as a primary diagnosis of dysplasia. Children from age 1 to 4 were most likely to be hospitalized with any diagnosis while children from 1 to 4 years and 10 to 17 years were equally likely to be hospitalized with a primary diagnosis of skeletal dysplasia. (Reference Table 7C.9 PDF [165] CSV [166])
Skeletal dysplasias as a primary diagnosis accounted for 0.3% of hospitalizations for any musculoskeletal diagnosis and 0.02% of hospitalizations for any condition. However, it is often the case that the primary diagnosis would reflect the problem associated with the condition rather than the condition itself. For example, with platyspondyly (flattened spinal bones), curvature of the lower back (lordosis) would be the diagnosis rather than dysplasia.
Total charges averaged $106,100 for a mean 10-day stay when hospitalized with a diagnosis of skeletal dysplasia with other medical conditions. With a primary diagnosis of skeletal dysplasia, the average stay was 8.6 days and cost $96,500. Mean length of stay and charges were highest in neonates. Total charges in 2013 were $144.8 million. (Reference Table 7C.9 PDF [165] CSV [166])
An estimated 300,000 children in the Unites States are diagnosed with juvenile arthritis or another chronic rheumatologic condition such as systemic lupus erythematosus, juvenile dermatomyositis, or linear scleroderma.1 These conditions generally require chronic care and, without appropriate treatment, can lead to significant disability.
Juvenile idiopathic arthritis (JIA) (formally called juvenile rheumatoid arthritis [JRA] or juvenile chronic arthritis [JCA]) is estimated to affect 1 in 1,000 children in the United States.2 JIA is diagnosed in a child younger than 16 years of age with at least six weeks of persistent arthritis. There are seven distinct subtypes, each having a different presentation and association to autoimmunity and genetics.3 Certain subtypes are associated with an increased risk of inflammatory eye disease (uveitis). Understanding the differences in the various forms of JIA, their causes, and methods to better diagnose and treat these conditions in children is important to future treatment and prevention. Among all subtypes, approximately half of children with JIA still have active disease after 10 years.4
There are several other causes of acute or chronic arthritis in children that do not meet the diagnostic criteria of JIA, including, but not limited to, rheumatic fever, Reiter syndrome/reactive arthritis, and the arthritis associated with inflammatory bowel disease.
Approximately 15% to 20% of cases of systemic lupus erythematosus (SLE) in the United States occur in children younger than 18 years of age. SLE is a chronic autoimmune condition characterized by the production of autoantibodies leading to immune complex formation and end organ damage. For reasons that remain unclear, pediatric SLE is associated with increased disease severity, increased short- and long-term morbidity, and mortality as compared to adult-onset SLE.5
Juvenile dermatomyositis is a chronic inflammatory condition characterized by inflammation of the skin and muscle. Estimated incidence of the disease in the United States is 0.5 per 100,000 people; the prevalence is not known.2
The sclerodermatous conditions are defined in part by the common clinical feature of tightening or hardening of the skin. Systemic scleroderma, also called diffuse cutaneous systemic scleroderma, is rare in childhood, accounting for only 2% to 3% of all cases of this condition, which has an estimated prevalence of 24 cases per 100,000 people. Linear scleroderma is the most common subtype of scleroderma diagnosed in the pediatric population. It is characterized by a linear streak of sclerosis typically involving an upper or lower extremity. 2
In 2006, the CDC Arthritis Program finalized a case definition for ongoing surveillance of pediatric arthritis and other rheumatologic conditions (SPARC) using the current ICD-9-CM diagnostically based data systems.6 In response to the variations in conditions that some felt should be included but were not, CDC generated estimates are not included in the case definition.
Rheumatologic conditions were diagnosed in 529,500 children and adolescent healthcare visits in 2013, of which 390,400 had a primary diagnosis of a rheumatologic condition. Only 2% of children and adolescents with any rheumatologic diagnoses were hospitalized (11,900), while less than 1% (3,700) with a primary diagnosis of a rheumatologic condition had a hospital discharge. Over one-half (58.3%) of children and adolescents with a rheumatologic condition diagnosis were seen in physicians’ offices. (Reference Table 7C.1.1 PDF [90] CSV [91] and Table 7C.1.2 PDF [96] CSV [97])
Females were hospitalized with a rheumatologic condition at nearly three times the rate of males, both for any diagnoses and as a primary diagnosis. As children age, there is a higher incidence of a rheumatologic condition diagnosis.
Any diagnoses of a rheumatologic condition accounted for 2.4% of hospitalizations for any musculoskeletal condition diagnosis, and 0.2% of all hospitalizations for any healthcare condition. Hospitalizations with a primary diagnosis of a rheumatologic condition were 0.7% of all musculoskeletal diagnoses and 0.1% of hospitalizations for any health condition diagnosis. (Reference Table 7C.10 PDF [171] CSV [172])
Total charges averaged $47,500 for a mean 5.1-day stay when children and adolescents were hospitalized with any diagnosis of a rheumatologic condition along with other medical conditions. With a primary rheumatologic diagnosis, the stay was shorter (4.4 days), and mean charges slightly lower at $42,500. Males as well as children 18 to 20 years old had slightly longer average hospital stays and average hospital charges. Total hospital charges for primary rheumatologic condition diagnosis discharges in 2013 were $157.3 million. (Reference Table 7C.10 PDF [171] CSV [172])
Many medical problems have musculoskeletal implications. This section discusses some of the more common of those diagnoses, including hemophilia, sickle cell disease, and endocrine and metabolic disorders such as rickets and lysosomal storage disorders.
Hemophilia is a genetic disorder characterized by abnormal blood clotting secondary to congenital deficiency of clotting factors VIII or IX. It may result in musculoskeletal problems by way of intramuscular hemorrhage and hemophilic arthropathy. Hemophilic arthropathy occurs through spontaneous bleeding into a weight-bearing joint, resulting in cartilage degeneration and arthrosis as well as asymmetric growth stimulation and deformity.
Sickle cell disease is inherited in an autosomal dominant fashion and is characterized by production of abnormal hemoglobin. This results in reduced oxygen delivery to tissues and can lead to multiple musculoskeletal manifestations, including painful bone infarcts, osteomyelitis, avascular necrosis, and vertebral compression fractures.
Metabolic bone diseases, such as rickets, occur due to abnormal calcium and phosphate metabolism. Rickets occurs in many forms, including vitamin D deficiency, vitamin D resistance, hypophosphatemia rickets, and renal osteodystrophy. Regardless of the cause, the result is inadequate calcification of bone and cartilage, resulting in bone pain and deformity.
The most common lysosomal storage disease is Gaucher’s disease, an autosomal recessive condition characterized by a deficiency in the enzyme beta-glucocerebrosidase. In Gaucher’s disease, there is an accumulation of glucocerebrosides, which contain glucose, in the tissues. This results in musculoskeletal manifestations that include bone deformity secondary to bone marrow infiltration, avascular necrosis, bone pain, pathologic fracture, and osteomyelitis.
Medical problems with musculoskeletal implications were diagnosed in 566,700 children and adolescent healthcare visits in 2013, of which 24% (134,000) had a primary diagnosis of a medical problem with musculoskeletal implications condition. One in ten children and adolescents with any medical problem diagnoses were hospitalized (54,700), while 3.5% (4,700) with a primary diagnosis had a hospital discharge. (Reference Table 7C.1.1 PDF [90] CSV [91] and Table 7C.1.2 PDF [96] CSV [97])
Males and females were hospitalized with a medical problem with musculoskeletal implications in about the same numbers, but with a primary diagnosis, males were more likely to be hospitalized. The highest rate of hospitalization, when compared to other MSK conditions, was for adolescents age 14 to 20 years of age.
Any diagnoses of a medical problem with musculoskeletal implications accounted for 10.9% of hospitalizations for any musculoskeletal condition diagnosis, and less than 1% of all hospitalizations for any healthcare condition. Hospitalizations with a primary diagnosis of a medical problem with musculoskeletal implications were less than 1% of all musculoskeletal diagnoses and 0.1% of hospitalizations for any health condition diagnosis. However, it is often the case that the primary diagnosis would reflect the problem associated with the condition rather than the condition itself. For example, a child with rickets is going to be hospitalized for a lower extremity deformity rather than for rickets. (Reference Table 7C.11 PDF [180] CSV [181])
Rickets accounted for 45.5% of all healthcare visits for medical problems with musculoskeletal implications, but 72% of the hospitalized cases. (Reference Table 7C.1.1 PDF [90] CSV [91])
Total charges averaged $119,200 for a mean 11.9-day stay when children and adolescents were hospitalized with any diagnosis of a medical problem with musculoskeletal implications along with other medical conditions. With a primary medical problem with musculoskeletal implications diagnosis, the stay was shorter (3.2 days), and mean charges about a fourth that of medical problems as a contributing condition ($29,500).
When hospitalized with any diagnosis of a medical problem with musculoskeletal implications along with other medical conditions, neonates and children less than 1 year of age had significantly longer stays and higher charges than other age groups, primarily due to cases of rickets. Total hospital charges for primary medical problem with musculoskeletal implications diagnosis discharges in 2013 were $138.7 million. (Reference Table 7C.11 PDF [180] CSV [181])
Musculoskeletal pain syndromes, including amplified musculoskeletal pain or juvenile fibromyalgia, chronic regional pain syndrome (reflex sympathetic dystrophy), benign hypermobility, and benign limb pains, are common diagnoses in the pediatric population. A systematic review examining the prevalence of chronic musculoskeletal pain found a range of prevalence rates between 4% and 40% in children. Rates were generally higher in girls and increased with age.1 It is estimated that 5% to 8% of new patients presenting to North American pediatric rheumatologists have a musculoskeletal pain syndrome.2
Amplified musculoskeletal pain can be localized or diffuse. Diffuse pain involving at least three major body parts for at least 3 months is seen in the diffuse type. Fibromyalgia is a subset of diffuse amplified musculoskeletal pain. Patients also typically have sleep disturbance and other somatic complaints, such as headaches and abdominal pain. Reflex sympathetic dystrophy (RSD), now called complex regional pain syndrome (CRPS), is a form of amplified pain in which autonomic dysfunction develops in an extremity, often following injury or trauma. The affected limb becomes swollen, discolored, and cold, and the area can be very painful with light touch (allodynia). The recommended treatment for these conditions includes restoring normal sleep patterns, a therapy program with a focus on exercise and desensitization, and cognitive behavioral therapy. Some patients require treatment in an in-patient setting. For further information see Childhood RND Educational Foundation, Inc., available at StopChildhoodPain.org [186].
Benign limb pains, sometimes referred to as “growing pains,” are most common in children age 2 to 5 years. Children with benign limb pains tend to complain of pain at night, often awaking from sleep due to pain. These symptoms tend to resolve with age.
Benign hypermobility is diagnosed in patients who have hypermobile joints3, without an underlying connective disuse disorder. This condition is common, affecting 8% to 20% of White populations. Anterior knee pain and back pain are more common in hypermobile vs non-hypermobile individuals.2
Pain syndromes were diagnosed in more than 2.7 million children and adolescent healthcare visits in 2013, of which 63% (1.8 million) had a primary diagnosis of a pain syndrome. Less the 1% of children and adolescents with any pain syndrome diagnoses were hospitalized (20,000), while a tiny fraction (1,700) with a primary diagnosis had a hospital discharge. Two-thirds (65%) of children and adolescents with a pain syndrome diagnosis were seen in physicians’ offices. (Reference Table 7C.1.1 PDF [90] CSV [91] and Table 7C.1.2 PDF [96] CSV [97])
Females were hospitalized with a pain syndrome diagnosis in slightly higher numbers than males, both for any diagnoses and as a primary diagnosis. Pain syndrome diagnoses increase as a contributing diagnosis in older children, but as a primary diagnosis was greatest between 14 and 17 years old followed by 5 to 13 years old.
Any diagnoses of pain syndrome accounted for just over 4% of hospitalizations for any musculoskeletal condition diagnosis, and 0.3% of all hospitalizations for any healthcare condition. Hospitalizations with a primary diagnosis of pain syndrome were 0.3% of all musculoskeletal diagnoses and a tiny portion of hospitalizations for any health condition diagnosis. (Reference Table 7C.12 PDF [189] CSV [190])
Total charges averaged $48,900 for a mean 6.1-day stay when children and adolescents were hospitalized with any diagnosis of a pain syndrome along with other medical conditions. With a primary pain syndrome diagnosis, the stay was shorter (3.1 days), and mean charges about half that of pain syndrome as a contributing condition ($25,800).
Sex was not a significant factor in length of hospital stay and average charges for a medical problem with a musculoskeletal pain syndrome diagnosis. In patients with a primary diagnosis of a pain syndrome, the average length of hospital stays (4.5 days) and average cost ($40,000) was highest among patients ages 10 to 13. Total hospital charges for primary pain syndrome diagnosis discharges in 2013 were $43.9 million. (Reference Table 7C.12 PDF [189] CSV [190])
An estimated 25,000 patients were seen in hospitals and emergency departments in 2013 for treatment of developmental dysplasia (DDH) of the hip.1 While DDH can often be successfully treated in childhood, it is now understood that even with successful treatment, residual effects can have a huge impact on the musculoskeletal burden of osteoarthritis in adulthood. One in four people are likely to develop symptomatic hip osteoarthritis in their lifetime.2 Thus, total hip arthroplasty is one of the most common musculoskeletal surgeries performed in the United States, with 343.6 thousand procedures performed in 2013.3 It is now also recognized that the underlying etiology of hip arthritis is often related to childhood developmental hip conditions such as Developmental Dysplasia of the Hip, Legg Calves Perthes disease, and Slipped Capital Femoral Epiphysis.4 A United States study of patients less than 50 years of age noted radiographic findings of developmental dysplasia of the hip in 23%.5. Long term outcome studies of surgeries to treat residual hip dysplasia in adults shows 74% native hip survival at 18 years.6 This long term impact of developmental hip conditions and the ability of hip hip preservation surgeries to prevent or delay onset of arthritis underscore the importance of early diagnosis and long term follow up into adulthood.
Other conditions commonly thought of as only affecting children, such as cerebral palsy, osteogenesis imperfecta, and spina bifida, are now being seen more than ever in adults thanks to the tremendous progress in care leading to longer life expectancy. Remarkably, some people with Duchenne’s muscular dystrophy are now surviving into early adulthood. Concomitant with this success has come a host of new issues concerning the transition of care to adulthood and the aging process.
Adults with these conditions are disproportionately affected by the aging process. Some issues are clear. For example, those with mobility challenges have difficulty participating in fitness regimens, leading to more sedentary lifestyles and secondary issues such as obesity, diabetes, and heart disease. Other issues are less clear. Adults with aftereffects of childhood musculoskeletal disorders have more difficulty accessing preventative care. Even more subtle are issues related to lack of providers skilled in treating adults with the sequela of childhood issues and psychosocial challenges.
The medical community needs to investigate whether the needs of patients are being met and if they are reaching full potential as productive adults. The margin of function which allows individuals to live independently is often very small. Early or more pronounced reduction in function associated with aging may make the difference in whether a care giver is required for activities of daily living or there is independent living.
Research into the Health-Related Quality of Life, prevalence of disease, potential to avoid disease, and availability of care, including preventative care, is required.
In 2013, total hospital charges for children and adolescents age 20 years and younger with a primary musculoskeletal-related diagnosis were $7.4 billion. Musculoskeletal trauma (41%) and deformity (37%) were the major contributors to total hospital charges, but all conditions contribute to the overall economic impact of musculoskeletal conditions in this age group. Furthermore, while musculoskeletal condition hospital charges represent 5.2% of total charges for all medical conditions for the age 20 years and younger age group, the number of discharges represent only 2% of total hospital discharges for any medical condition in this age group, indicating that musculoskeletal conditions may be more expensive to treat than many other childhood conditions. (Reference Table 7C.13 PDF [195] CSV [196])
It is important to note that the overall cost of musculoskeletal conditions in the 20 years and younger population is much greater than just hospital charges. First, the $7.4 billion includes only hospitalizations with a primary, or first, diagnosis in the databases, representing less than 2% of 2013 healthcare visits with any musculoskeletal condition diagnosis. Not included in this burden are expenditures for visits to emergency departments, outpatient clinics, and physicians’ office, as well as other medical care expenditures such as physical therapy, rehabilitation, and medications.
While gender is not a factor in the distribution of hospital charges, age is a major contributor. Children in the middle years of childhood, especially ages 10 to 13 years, have a higher share of total hospital charges (16%) due to musculoskeletal conditions than any other age group. Musculoskeletal condition hospital charges are also a higher share for those age 14 to 17 years (14%) and ages 5 to 9 years (8.6%).
To fully understand the burden of musculoskeletal diseases on children and adolescents, it is mandatory that data be available on prevalence, healthcare needs, cost associated with treatment, limitations due to musculoskeletal conditions, and overall impact these conditions have on the lives of children and adolescents. The HCUP KID, HCUP NIS, and HCUP NEDS databases provide a tremendous asset in understanding hospitalizations for this analysis, but they, too, have limitations. First among these is the inability to truly determine primary cause for visits, as multiple diagnosis codes may be included with each record, with no way of knowing which is the primary diagnosis. In addition, many healthcare visits are to a physician’s office, and the database for these visits National Ambulatory Medical Care Survey (NAMCS) is small and often contains insufficient cases (<35) for reliable analysis even when merging several years of data. This is particularly true for the very young patients (0 to 5 years) and for rare conditions. Injuries occur in enough numbers that this is not a problem. However, many other conditions had low numbers.
A second key challenge is ensuring that children with chronic medical and musculoskeletal problems have access to care, particularly for those with Medicaid or other government-funded insurance. Low physician reimbursement by government insurance results in fewer physicians who are willing or able to care for these patients, making access to needed specialty care difficult. Additionally, pediatric subspecialists who take care of musculoskeletal conditions are typically located at large children’s hospital in more populous cities, further reducing access to care for those in rural areas. Because of the unique nature of pediatric musculoskeletal problems and treatments, many adult subspecialists who may be more accessible are unable or unwilling to treat pediatric patients.
A third challenge is the need to track pediatric patients into adulthood to determine lifelong burden of their pediatric musculoskeletal disease. Once a child turns 18 years, the system loses them as they become more mobile and move on to other caregivers. Further, they may lose parental insurance or their Medicaid coverage. A better way to obtain long-term follow-up on their history and long-term outcomes of treatment of pediatric musculoskeletal disease is needed.
Poor bone health is being recognized as a key problem in pediatric musculoskeletal disease, one that will last a lifetime. Key factors leading to poor bone health are Vitamin D deficiency and childhood obesity. The current healthcare data system makes it very difficult to quantify the burden of these problems because they are infrequently evaluated as the primary diagnosis. Additionally, patients are rarely admitted or discharged for treatment specific for these diagnoses. In the future, methods for estimating the incidence of these diagnoses more accurately and assessing their contribution to musculoskeletal disease is necessary. Education of the individual, family, and society about the burden of obesity and Vitamin D deficiency is necessary to improve overall bone health in the United States.
Quality of life assessments in children and adolescents that allow better measure of the personal impact of pediatric musculoskeletal disease is lacking. In assessment of musculoskeletal disease for adults, lost wages and lost workdays are used to quantify burden. There is no corresponding way to measure burden in children. Currently, it is quantified indirectly by measuring lost wages and lost workdays for the child’s caregiver. Better methods for quantifying indirect burden of pediatric musculoskeletal disease is needed.
Better long-term follow-up data on pediatric musculoskeletal conditions is needed. Once patients reach adulthood, it becomes difficult for the physician who cared for their musculoskeletal conditions to keep track of them. This results in difficulty understanding adult manifestations of pediatric musculoskeletal conditions. On a global basis, the disability-adjusted life year (DALY), developed in the 1990s as a way of comparing the overall health and life expectancy of different countries, is used as a measure of overall disease burden expressed as the number of years lost due to ill-health, disability or early death. Disabilities incurred in childhood, expressed in the DALY, would provide greater understanding of the lifelong burden of these conditions.
MUSCULOSKELETAL INFECTIONS
Osteomyelitis: 730.0, 730.1, 730.2, 730.8, 73090, 73091, 73092, 73093, 73094, 73095, 73096,73097
Septic arthritis: 711.0, 711.4
Soft tissue infections (infective myositis): 72800, 72886
Lyme disease: 08881
Tuberculosis: 015
DEFORMITY
Upper Extremity:
Polydactyly: 75500, 75501
Syndactyly: 75510, 75511, 75512
Reduction deformities: 755.2
Other congenital anomalies upper limb: 755.5, 736.0, 736.1, 736.2, 73690, 75489, 75681, 75689
Lower Extremity:
Polydactyly: 75502
Syndactyly: 75513, 75514
Reduction deformities: 755.3
Other congenital anomalies lower limb: 755.6
Congenital deformities: 754.4, 754.59, 754.6, 754.7, 72781, 73400, 736.7, 736.8
Hip and Pelvis:
Congenital deformity of hip joint: 75561, 75562, 75563
Hip joint acquired: 736.3, 73220, 73860
Developmental dysplasia: 754.3
Spine and Pelvis:
Of spinal cord: 742.5
Of vertebral column: 737, 73850, 73200, 75420, 756.1
Other and Unspecified:
Congenital deformities: 754.8, 75540, 75580, 75590, 75682, 75690, 75689
TRAUMA: Fractures, dislocation, sprains and strains, open wound, crushing injury, contusion, traumatic compartment syndrome, unspecified injuries, injuries to nerve roots and spinal plexus
Upper Extremity: 810, 811, 812, 813, 814, 815, 816, 817, 818, 819, 831, 832, 833, 834, 840, 841, 842, 880, 881, 882, 883, 884, 885, 886, 887, 90520, 923, 927, 95891, 95920, 95930, 95940, 95950
Lower Extremity: 820, 821, 822, 823, 824, 825, 826, 827, 828, 829, 836, 837, 838, 844, 845, 90530, 90540, 924, 928, 95960, 95970, 95892
Hip and Pelvis: 835, 843, 84850, 808, 959.1, 953
Spine and Trunk: 805, 806, 807, 846, 847, 809, 875, 876, 90510, 92200, 92210, 92230, 92231, 92232, 92233, 92280, 92290, 926.1, 92680, 92690, 952
Birth Trauma: 767
Child Abuse: 995.5
NEUROMUSCULAR CONDITIONS
Cerebral palsy (CP): 343
Spina bifida (SB): 741
Muscular dystrophy (MD): 359
Charcot-Marie-Tooth disease (CMT): 35610, 35620
Other: 334, 335, 33600, 336
SYNDROMES WITH MUSCULOSKELETAL IMPLICATIONS
Marfan sydrome/Ehlers Danlos syndrome/other connective tissue disorders: 75982, 75683
Down's syndrome: 75800
Neurofibromatosis (NF): 23770, 23771, 23772
SPORTS INJURIES (Sports injuries data is from NEISS and does not use ICD-9 codes.)
SKELETAL DYSPLASIAS
Dysplasias: 75989, 65580, 73399, 39330
Chondrodystrophy/achondroplasia/hypochondroplasia: 75640
Dwarfism (thanatophoric dysplasia): 25940, 75651
Congenital absence rib: 75630
Osteogenesis imperfecta: 75651
Osteopetrosis: 75652
Other: 75654, 75655, 75656, 75659
NEOPLASMS
Benign:
Benign lesion of bone/cartilage: 213
Lipoma: 21400, 21410, 21420, 21430, 21480, 21490
Benign lesion of CT/ST: 215
Malignant:
Malignancy of bone/cartilage: 170
Malignancy of CT/ST: 171
RHEUMATOLOGIC CONDITIONS
Rheumatic fever: 39000, 39092
Reactive arthritis/Reiter disease (underlying disease, no principal diagnosis): 711.1
Juvenile idiopathic arthritis: 714.3
Ankylosing spondylitis and inflammatory spondylopathies: 720
Psoriatic arthritis: 696
Arthropathy of inflammatory bowel disease: 71310
Systemic lupus erythematosus: 71000
Juvenile dermatomyositis: 71030
Localized scleroderma: 70100, 71010
MEDICAL PROBLEMS WITH MSK IMPLICATIONS
Hemophilia: 00286
Sickle cell: 28260
Endocrine and metabolic disorders: 75650
Gaucher disease (lipidoses/lysosomal storage disorders): 27270
Osteoporosis: 733.0
Hyperthyroid (thyrotosicosis w/wo goiter): 242
Rhabdomyolysis: 72888
Other conditions: 28610, 25890
Rickets (Vitamin D deficiency, phosphorus, and calcium metabolism disorders): 268, 275.3, 275.4
PAIN SYNDROMES
Amplified musculoskeletal pain/Juvenile primary fibromyalgia syndrome: 30789, 72910
Reflex sympathetic dystrophy (complex regional3722 pain syndrome/CRPS): 337.2
Benign hypermobility/hypermobility syndrome: 72850
Benign limb pains (“growing pains”): 719.4
Racial and ethnic disparities in healthcare have been well established in the literature. Reasons for disparities include cultural beliefs, socioeconomic differences, language barriers, and discrimination or bias in the healthcare system. A 2003 IOM report confirmed racial and ethnic minorities receive lower quality healthcare and have poorer outcomes than their Caucasian counterparts.1 A 2019 Medicare study found significantly decreased rates of surgical intervention among racial and ethnic minorities.2 The US Census Bureau reported that minorities comprised 38.4% of the population in 2015; by 2050, it is projected that non-Hispanic whites will no longer be the majority group in the United States.3 The increasing minority population has hastened the need to define, understand, and reduce these differences. Furthermore, disparities in healthcare and health outcomes will become increasingly economically burdensome.
Disparities in musculoskeletal care has been a topic of increased interest in the past decade. Schoenfeld et al et al reported that racial and ethnic minorities are at increased risk of complications and/or mortality after orthopaedic surgical intervention.4 Several other studies have demonstrated decreased utilization and access to orthopaedic care among minorities, such as joint replacement and spinal surgery.
Race and ethnicity also have an impact on the prevalence of certain musculoskeletal conditions. Ankylosing spondylitis and osteoporosis are examples of conditions for which ethnicity is a strong risk factor. Further research is needed to determine whether several other musculoskeletal conditions are impacted by ethnicity.
Racial/ethnic groups in the BMUS report are defined based on the major databases analyzed for this report. The six groups included in the databases are white, black, Hispanic, Asian/Pacific Islander, Native American, and other. Hispanic ethnicity applies to persons of all races, therefore they are identified by their ethnicity, while other persons are defined as non-Hispanic white, non-Hispanic black, and non-Hispanic other. Due to small sample sizes, non-Hispanic other persons include Asian/Pacific Islander, Native American, and others.
To broaden the scope of ethnic and racial differences and disparities, a literature search was also conducted. Findings on the impact of musculoskeletal diseases on more specific races and ethnic groups are discussed by condition. Data findings are presented to provide a snapshot of differences and disparities. However, the NEDS database, the largest database, does not include a race/ethnicity variable, thus the importance of differences in emergency department visits is unavailable.
Non-Hispanic white persons report experiencing more musculoskeletal conditions in the previous year than members of other racial/ethnic groups. In 2015, 54.5% of non-Hispanic white persons reported they had at least one musculoskeletal condition, compared to 46.4% of non-Hispanic blacks, 38.6% of non-Hispanic others, and 40.6% of persons of Hispanic ethnicity. Chronic joint pain was the most frequently mentioned condition among all persons, with knee pain the most common joint. Non-Hispanic black persons reported back pain radiating down the leg (11.0%) at nearly the same rate as non-Hispanic white persons (11.4%), and a slightly higher rate of carpal tunnel syndrome (4.2% vs. 3.5%). (Reference Table 7D.1 PDF [203] CSV [204])
The burden of musculoskeletal conditions can be defined in economic terms or as it affects those suffering from these conditions. In this section, burden is defined in terms of limitations in activities of daily living (ADL) and as bed or lost work days.
Approximately half of persons reporting musculoskeletal conditions also report they suffer limitations in ADL as a result of these conditions. Among non-Hispanic white persons, 28.7% reported limitations, 24.8% of non-Hispanic black persons have limitations, 18.7% of non-Hispanic other persons, and 18.6% of those of Hispanic ethnicity. Arthritis and back/neck problems are the most common conditions causing limitations. (Reference Table 7D.1 PDF [203] CSV [204])
Bed and Lost Work Days
Non-Hispanic black persons report the highest number of bed days in the previous year due to musculoskeletal conditions (24.7 days on average), while those of Hispanic ethnicity lost, on average, the most work days (14.3 days). (Reference Table 7D.1 PDF [203] CSV [204])
Racial disparities in the prevalence of spinal conditions have sparsely been discussed in the literature. Most spinal deformities, including scoliosis, have the higher rates of diagnosis in Caucasians (BMUS). A 2011 retrospective study of patients over 40 years old revealed a prevalence of almost twice the rate of scoliosis in whites compared to African-Americans (AA).1 Specific to adolescent idiopathic scoliosis (AIS), African-American patients had higher curvatures at presentation compared with whites and Hispanics. Therefore, they were more likely to have surgery as their initial treatment.2 For this reason, AA patients may need to present at an earlier age for screening. There is a general belief that genetics play a role in progression of AIS; however, it is unknown which genetic factors or whether race is involved.
Lumbar radiculopathy is a spinal nerve root condition caused by nerve compression, inflammation, or injury in the lumbar spine. In a database study of a young, military population, lumbar radiculopathy was found to be more common among white patients.3 Lumbar spinal stenosis, a narrowing of the spinal canal resulting in nerve compression, is another major cause of low back pain and nerve symptom. Overall, hospitalizations for this condition have been reported to be much more common in whites. Blacks and Hispanics have lower rates of surgical hospitalization for lumbar spinal stenosis than did whites.4 Cultural barriers and attitude toward surgery may be responsible for this difference. In patients undergoing surgery for lumbar stenosis, blacks have higher complication rates, longer hospital stays, less likelihood of discharge home, and short preoperative and postoperative follow-up.5,6 African-American patients also accrue higher hospital-related costs and are prescribed fewer medications.5 Cervical spine surgeries have also been analyzed. African-American patients have higher rates of in-hospital complications and mortality than other ethnicities.7 Much of this difference was likely due to socioeconomic status, insurance status, and access issues.
Ankylosing spondylitis (AS) is an inflammatory arthritis that primarily affects the spine. AS is highly associated with the HLA B27 gene. AS is three times more common in whites than in blacks.8 This is mostly due to the lower prevalence of HLA-B27 in individuals of African descent. A prospective study by Jamalyaria et al compared the disease severity of AS in different ethnic groups. They determined that African-Americans, and Hispanics to a lesser degree, have greater functional impairment, higher disease activity, and greater radiographic severity compared to whites.9 The reason for this could not be determine; however, access to care and genetics are potential factors.
There is conflicting data related to racial differences in back pain. Hootman and Strine reported on 3-month prevalence rates of neck and back pain and found a higher prevalence in whites than other ethnic groups.10 Knox et al analyzed the rates of low back pain in military service members resulting in a visit to a health care provider and reported the highest incidence rates among African-Americans.11 The reason behind the variability is unknown; however, there is believed to be a genetic component. Importantly, a survey study found no significant differences in care-seeking behavior between racial groups for acute or chronic low back pain.12
Non-Hispanic white persons report experiencing neck/cervical and lumbar/low back pain at slightly higher rates in the previous year than members of other racial/ethnic groups. However, back pain with radiating leg pain was reported at similar rates among all racial/ethnic groups except for other/mixed non-Hispanic persons, who reported a lower rate.
In 2015, 36.4% of non-Hispanic white persons reported back pain, compared to 31.0% of non-Hispanic blacks, 30.3% of persons of Hispanic ethnicity, and 26.4% of non-Hispanic others. Low back/lumbar pain was mentioned about twice as often as neck/cervical pain. Respondents are asked if they have radiating leg pain only if the identify suffering from low back pain. Approximately one-third of persons with low back pain also reported radiating leg pain, with non-Hispanic black persons highest (39%) and non-Hispanic other/mixed persons lowest (34%). (Reference Table 7D.2 PDF [213] CSV [214])
Non-Hispanic whites (18.4 per 100 persons) were slightly more likely to seek healthcare for treatment of low back/lumbar pain in 2013 than non-Hispanic blacks (17.1/100) and Hispanics (14.2/100). However, non-Hispanic others/mixed race were much less likely to seek healthcare for back pain (7.3/100). Rates for non-Hispanic whites (6.2/100) and non-Hispanic others (6.0) were similar for healthcare visits for neck/cervical pain, but lower for non-Hispanic blacks (4.5) and Hispanics (3.0). (Reference Table 7D.2 PDF [213] CSV [214])
Non-Hispanic black persons report the highest number of bed days in the previous year due to back pain (8.7 days on average), while those of Hispanic ethnicity lost, on average, the most work days (14.1 days). (Reference Table 7D.2 PDF [213] CSV [214])
Research starting in the late 1980s and extending to 2011 shows a consistent pattern of doctor-diagnosed arthritis prevalence among races and ethnicities, although prevalence rose among all groups. Persons of Hispanic ethnicity and Asian/Pacific Islanders have lower arthritis prevalence than non-Hispanic whites, non-Hispanic blacks, and non-Hispanics of other races. However, a study of the 2013 Behavioral Risk Factor Surveillance Survey (BRFSS) participants residing in Hawaii of health disparities of Native Hawaiians and Pacific Islanders (NHPI), Whites, and Asians found that NHPI males had a significantly higher prevalence of arthritis, which peaked twenty years earlier, than White and Asian males. The prevalence of arthritis peaked at 65-79 years in males and females in all racial groups, except NHPI males where it peaked at 45-54 years. At the NHPI peak age range, arthritis prevalence was 49.4% among NHPI males compared to White males (222.2%) and Asian males (17.9%). No significant differences were found among females.1
American Indians/Alaska Natives higher than non-Hispanic blacks and resembling non-Hispanic whites.2,3 A 2009-2011 study of prevalence rates among females only reported the same pattern, but with higher rates than found in both sexes.4 Arthritis-attributable activity limitation, arthritis-attributable work limitation, and severe joint pain were found to be higher for non-Hispanic blacks, Hispanics, and multiracial or other respondents with arthritis compared with non-Hispanic whites with arthritis.3
Osteoarthritis, or degenerative joint disease, is the most common form of arthritis. The incidence of osteoarthritis in different ethnicities is similar. Disabling OA is at least as prevalent among African Americans and Hispanics as among non-Hispanic whites.5 African-Americans, however, report greater pain and activity limitation in comparison to Caucasians.6,7,8 African-Americans have higher prevalence of knee symptoms, radiographic knee osteoarthritis, and symptomatic knee osteoarthritis than whites,8 and 77% more likely to have knee and spine osteoarthritis together.9 Hispanics are 50% more likely than non-Hispanic Whites to report needing assistance with at least one instrumental activity of daily living and report difficulty walking.10 Prevalence of osteoarthritis of the knee is on the rise, due in part to the growing epidemic of obesity. Hispanic and African-American women have disproportionatly high rates of obesity leading to higher rates of knee osteoporosis, with subsequent quality-adjusted life-years losses, than found among Caucasian women.11
There is evidence of race-based differences in rheumatoid arthritis (RA). Ethnic variations have been found in the clinical expression of RA, both in the frequency and types of SE-carrying HLA–DRB1 alleles,12 with non-Hispanic whites having the lowest percentage of rheumatoid factor positive results.13 Hispanics exhibit more tender and swollen joints than non-Hispanic whites, while African-Americans are slightly older at onset.12 African American and Hispanic patients have higher disease activity level, lower rates of remission, and worse functional status than white patients, in spite of more aggressive treatment strategies in recent years.13,14 There are also differences in utilization of disease-modifying anti-rheumatoid drugs (DMARDs), the gold standard treatment of RA. Certain studies suggest that treatment differences may be related to patient preference. Constantinescu et al found that fewer African-American patients preferred aggressive treatment compared to white patients with similar disease severity.15 This study suggests that improvement in patient literacy about rheumatoid arthritis could decrease the disparity in management.
Gout, one of the most common forms of inflammatory arthritis, is characterized by severe joint pain and destruction. A population-based cohort study demonstrated that African-Americans were at an increased risk of gout.16 African-Americans with gout have also been found to function worse than their Caucasian counterparts.17 Another database study found that African-Americans with gout were less likely to receive urate-lowering therapy with allopurinol.18 Studies have shown a similar efficacy of ULT between black and white patients.19,20 These results suggest that decreasing the disparity in gout treatment will improve disease severity in African-Americans.
Ethnic disparity has been widely studied in SLE, with findings that West-African immigrants experience SLE (lupus) more than those native to Europe or America, with many having the condition before migration. A San Francisco study found SLE was four times higher in African-American women than in Caucasian women. Among Asians, SLE is reported to be more frequent among Chinese settling outside China.21
Total hip and knee replacements, generally indicated for end-stage arthritis, are two of the most common and successful major surgical procedures performed in the United States. Outcomes after total joint replacement are similar between black and white patients after controlling for socioeconomic factors.22 Unfortunately, racial disparities in the utilization of these procedures has been demonstrated in multiple studies. A Medicare database study by Singh et al demonstrated that blacks are less likely to receive joint replacement surgery compared to whites. Importantly, the utilization disparity did not improve over an 18-year period. Blacks also had inferior outcomes including longer hospital stays and higher rates of readmission.23 Another prospective study revealed that blacks are less likely to receive a recommendation for joint replacement surgery; however, this difference appeared to be related to patient treatment preference.24 African American patients are also less familiar with TKA than their white counterparts and more likely to anticipate greater perioperative pain and longer recovery.25,26 Thus, patient education about the procedure is likely a major factor that will increase utilization of joint replacement procedures by African-Americans.
In 2015, non-Hispanic whites and Non-Hispanic blacks self-reported doctor-diagnosed arthritis (told be a doctor they have arthritis) at similar rates (22.6/100 persons and 22.2/100, respectively), while persons of Hispanic ethnicity reported a lower rate (15.4/100). Persons of non-Hispanic other/mixed race did not report in sufficient numbers to be cited. (Reference Table 7D.4.1 PDF [225] CSV [226])
The numbers reported for arthritis-attributable activity limitations as a total followed the same pattern as doctor-diagnosed arthritis, with non-Hispanic whites and non-Hispanic blacks similar (11.1/100, 10.9/100, respectively), Hispanics much lower (5.7/100), and insufficient numbers of non-Hispanic others/mixed race to be cited. However, by specific type of limitation, non-Hispanic blacks report higher rates than other racial/ethnic groups. (Reference Table 7D.4.1 PDF [225] CSV [226])
In 2015, those of Hispanic ethnicity reported more lost work days due to arthritis, on average, than other racial/ethnic groups. (Reference Table 7D.4.2 PDF [233] CSV [234])
Considering only hospitalizations with an arthritis diagnosis, in 2013, non-Hispanic blacks had a slightly higher rate (2.8/100 persons) than non-Hispanic whites or Hispanics (2.6/100), with non-Hispanic others/mixed race much lower (1.1/100). The HCUP NEDS (emergency department) database does not report race/ethnicity, hence no numbers are available for other types of healthcare visits. Non-Hispanic blacks also had slightly longer hospital stays with an arthritis diagnosis. (Reference Table 7D.4.2 PDF [233] CSV [234])
Joint replacement is a common procedure performed to alleviate the pain from arthritis. As noted above, the literature reports lower rates of hip and knee procedures among non-Hispanic blacks. This finding is supported by the rates of all arthroplasty procedures performed in hospitals in 2013. Non-Hispanic white persons received 80% of hip replacements and 77% of knee replacements compared to the 62% of the population they represented. All other racial/ethnic groups had small shares of procedures than they represented in the population. (Reference Table 7D.4.3 PDF [241] CSV [242])
Race and ethnicity are important factors in the incidence of osteoporosis. The World Health Organization defines osteoporosis as a T score less than -2.5.1 African-Americans tend to have higher bone mass levels than Caucasians and Asians.2,3 In adults 50 years of age and older, approximately 10% of non-Hispanic white women have osteoporosis, compared with 6% of non-Hispanic black women and 10% of Hispanic women. It is estimated that an additional 50% of non-Hispanic white and Asian women have osteopenia, compared with 39% of black women and another 38% of Hispanic women.4,5
Osteoporotic fractures are a major health care concern due to their morbidity and mortality along with health expenditures. In 1995, the estimated health care costs associated with osteoporotic fractures was 13.8 billion.6 Decreased bone strength predisposes patients to an increased risk of fragility fractures, especially hip fractures. African-Americans have the lowest rates of hip fractures since they have the highest bone density.7 In a database study, Cheng et al reported that among traditional Medicare beneficiaries with fractures, osteoporosis was diagnosed nearly twice-as-often for white women compared with black women across all age groups.8 Ethnicity and race influenced the risk of fracture even after adjusting for multiple variables. Overall, the risk of fracture was 49% lower among African American women than among white women.9 Longer hip axis lengths have also been linked to an increased risk of hip fracture and hip axis lengths are reportedly shorter among African Americans and Asians, even after adjusting for height.10 African-American women who sustain an osteoporotic fracture, unfortunately, experience higher morbidity and mortality in comparison.11 This is possibly due to differences in hospital volume or it could reflect variations in care.
Race and ethnicity also are important factors in the screening and treatment of osteoporosis. In a retrospective review, Curtis et al found a significant disparity in recommendation for osteoporosis screening between AA and white women. Among Medicare enrollees, 33% of white women have screenings for BMD, but only 5% of African American women have such screenings.12 Among women with fractures, African Americans had a lower likelihood of both BMD testing and treatment.10,13 Hamrick et al reported that while 80% of white women received pharmacotherapy after osteoporosis diagnoses, only 68% of black women did.14 A cross sectional study by Curtis et al showed that African Americans are significantly less likely than Caucasians to receive osteoporosis medication. Minority women are less likely to receive hormone replacement therapy.15
In 2013, 892,600 patients discharged from hospitals in the US had a primary (first) diagnosis of osteoporosis. The distribution of persons with a primary diagnosis of osteoporosis did not reflect other data that indicates lower rates of osteoporosis among non-Hispanic blacks and higher rates among non-Hispanic whites and those of Hispanic ethnicity. Among this group, only 7% were classified as non-Hispanic whites.
Within the same time period, 540,600 patients were discharged with a fragility fracture diagnosis, and may or may not have had a diagnosis of osteoporosis. Among those with a fracture diagnosis, 82% were non-Hispanic white persons, with all other racial/ethnic groups accounting for only 4%-5% of discharges with a fracture. (Reference Table 7D.5 PDF [250] CSV [251])
The differences in hospital discharges for osteoporosis and fragility fractures from known prevalence rates may reflect treatment rates among racial/ethnic groups and coding of fractures before the underlying cause of the fracture in medical records, particularly among non-Hispanic white patients.
There is a paucity of literature regarding racial differences in sports-related injuries. Anterior cruciate ligament rupture is a common sports injury. A retrospective study of women’s professional basketball players over a 4-year span reported a higher rate of ACL tears in white players than their African-American counterparts.1 A difference in femoral morphology has been found between racial groups and may be a contributor to the potential difference in ACL injury rates.2 Another significant sports injury, lower extremity tendon ruptures, was analyzed in a military database study. Quadriceps, patellar, and Achilles tendon ruptures were examined. African-American service members had a significantly higher rate of lower extremity tendon rupture when compared to white service members.3 A biomechanical study showed a higher Achilles tendon stiffness in black athletes which potentially makes them more susceptible to rupture.4
Ankle sprains are the most common injury in athletic populations.5 Both AA and white races have a higher rate of ankle sprains than Hispanics.6 This is potentially due to the difference in type of athletic activities, for example soccer vs basketball.
Falls are an important cause of hospital admission and can lead to injuries such as hip and distal radius fractures. Whites have a higher incidence of falls than African-Americans.7 In a prospective study, Kiely et al also found a higher rate of falls in whites; however, after adjusting for confounding variables including types of activity and community characteristics, the difference was minimized.8 According to a retrospective study by Strong et al, in patients 65 and older admitted for falls, AA patients have a higher risk of mortality after discharge from the hospital.9 This highlights the need for improved follow-up after discharge.
African-Americans have a lower overall incidence of fractures than whites.10,11; however, there is minimal research on fracture risk other than in the hip. Much of this is related to higher bone density in blacks along with the difference in activities engaged in. Some studies have also investigated for disparities in the management of fractures. Opel et al found that after adjusting for insurance status and severity of injury, African-Americans had significantly lower odds of receiving surgical treatment for humeral shaft fractures than white males.12 The results suggested a possible bias in treatment decision-making, leading to less aggressive management in African-Americans.
Non-Hispanic whites self-report the highest rate of injuries (3.3/100 persons) for which they sought medical care in 2013-2015. Non-Hispanic other/mixed race persons reported the lowest rate 1.3/100). (Reference Table 7D.6 PDF [254] CSV [255])
Data for both self-reported injuries for which medical care was sought and for hospital discharges support research findings reported above. Non-Hispanic white persons represented two-thirds or more of reported injuries from falls, trauma, or other causes, but were a smaller share of trauma accidents than falls or other causes.
Among hospital discharges for injuries, 55% of non-Hispanic white persons were hospitalized due to a fall, compared to 33% of non-Hispanic black persons. Persons of Hispanic ethnicity had the highest share of discharges due to trauma injuries (34%) , followed closely by non-Hispanic blacks (31%). (Reference Table 7D.6 PDF [254] CSV [255])
Primary sarcomas represent the least common malignancies in bone, although osteosarcoma represents the most common nonhemoapoietic primary tumor of bone. Osteosarcoma is a primary malignant bone-producing tumor. In a review by Ottaviani, osteosarcoma had a higher incidence in African-Americans (AA) (6.8 per million persons per year] and Hispanics (6.5 per million) than in whites (4.6 per million).1 The reason for a potential higher incidence in blacks may be due to genetic factors, but it has not been determined.
Ewing sarcoma is a malignant tumor of bone and soft tissue. Race is an important factor in the incidence of ES, with Caucasians more likely to develop ES than African Americans or Asians. In a database study, Worch et al showed that ES is 8 times more likely to occur in the white population compared with African Americans and 1.9 times more likely to occur in the white population compared with Asian-Americans and Native Americans.2 Another database study by Worch et al., however, showed overall survival was significantly worse for patients. These results suggest a genetic component to the disease.3
Soft tissue sarcomas are the sixth most common primary cancer among young adults and adolescents aged 15-29.4,5 Musculoskeletal tumors included in this group include rhabdomyosarcoma, synovial sarcoma, and liposarcoma. Hsieh et al. showed that AA had the highest incidence rates of fibromatous neoplasms, rhabdomyosarcoma, and Kaposi sarcoma among all racial/ethnic groups. This study also revealed that Hispanic males and females had significantly higher liposarcoma rates than other racial/ethnic groups.6 A database study by Alamanda et al found that African Americans encounter death due to soft tissue sarcomas at a much larger proportion and faster rate than their respective white counterparts. African Americans frequently presented with a larger size tumor, do not undergo surgical resection, or receive radiation therapy as frequently as compared with their white peers.7,8
Multiple myeloma is a cancer of plasma cells and is the most common malignancy arising in bone. Multiple myeloma (MM) is the most common hematologic malignancy among blacks in the US and the second most common hematologic malignancy in the country.9 A large database study concluded that blacks have an earlier onset and a higher incidence of MM This study also found African-Americans to have better survival rates, which is different than most conditions found in the literature.10 These results suggest a different disease biology. Fiala et al performed a database study regarding racial disparities in multiple myeloma treatment. After controlling for overall health and potential access barriers, black patients were found to be 37% less likely to undergo stem cell transplantation, and 21% less likely to be treated with bortezomib, an antineoplastic agent which is considered the gold standard in chemotherapy treatment of MM. Moreover, the authors found that the underuse of these treatments was associated with an increase in the incidence of death among black patients.11 The difference in treatment may be due to patient preference, patient education, or implicit biases in management. More research is needed to examine these factors.
Incidence of musculoskeletal cancers is reported in BMUS based on data published by the National Cancer Institute’s Surveillance, Epidemiology and End Results Program (SEER). Data is shown for bones and joints cancers but is not broken down for specific types of sarcomas. Myeloma (multiple myeloma) is a cancer of plasma cells in the bone marrow. Based on SEER data 2010-2014, non-Hispanic whites have a higher incidence of bones and joints cancers than do non-Hispanic blacks and those of Hispanic ethnicity, but all have very low incidence. Non-Hispanic white males had the highest incidence at 12 cases per one million persons. Myeloma has a higher incidence, with non-Hispanic blacks higher than non-Hispanic whites. Incidence was not reported for those of Hispanic ethnicity. SEER reported death rates for musculoskeletal cancers follow the same pattern as incidence rates but are much lower. (Reference Table 7D.7 PDF [262] CSV [263])
The impact of race and ethnicity on the etiology and management of musculoskeletal conditions requires more extensive investigation. The influence of race and ethnicity on the incidence of musculoskeletal conditions may be due to genetics along with difference in activities participated in. Genetic differences, however, have not been well defined in the vast majority of conditions. Clarifying this may lead to advancements in the management of certain conditions including osteoporosis, multiple myeloma, and spinal deformities.
The difference in incidence is also largely influenced by the lower rate of presentation by ethnic minorities to a physician. We also need to enhance awareness of any disparities in the management of musculoskeletal conditions. Race-based differences in the treatment of certain conditions may indicate an inherent bias. They may also be related to access issues and patient perception. The treatment of disabling osteoarthritis is a good example. Osteoarthritis has been found to be as prevalent in AA and Hispanic populations as in non-Hispanic white populations. Several studies, however, have shown that minorities undergo joint replacement procedures at a significantly lower rate. Ethnic minorities are less familiar with certain surgical procedures. Also, certain primary care physicians are less likely to refer patients to surgeons for consultations depending on their access to these services or their perception of what their patient's insurance may allow for. Unfortunately, AAs may have a higher rate of adverse outcomes.1 The reasons for this disparity are multifactorial but include less familiarity and lower expectations with the procedure in minority populations. Also, minorities tend to have procedures at lower volume hospitals which may contribute to more adverse outcomes.
Lastly, access to adequate postoperative care should be considered in adverse outcomes, be it from another family member that can afford to miss workdays or certain ancillary services provided to the patient.
A greater awareness regarding the disparities in musculoskeletal conditions and their management is needed. Further research into the reasons for differences in incidence of certain conditions will allow for better and possible earlier intervention. Moreover, enhanced understanding and defining the causes of racial disparities in the management of musculoskeletal diseases will allow improved and more equitable care in an increasingly diverse population.
Links:
[1] https://www.census.gov/newsroom/press-releases/2018/cb18-41-population-projections.html
[2] https://www.rand.org/pubs/tools/TL221.html
[3] https://www.cdc.gov/chronicdisease/about/costs/index.htm#ref1
[4] https://bmus.latticegroup.com/docs/bmus_4e_t7b.1.pdf
[5] https://bmus.latticegroup.com/docs/bmus_4e_t7b.1.csv
[6] https://bmus.latticegroup.com/file/bmus4eg7b11png
[7] https://bmus.latticegroup.com/docs/bmus_4e_g7b.1.1.png
[8] https://bmus.latticegroup.com/file/bmus4eg7b12png
[9] https://bmus.latticegroup.com/docs/bmus_4e_g7b.1.2.png
[10] https://bmus.latticegroup.com/file/bmus4eg7b13png
[11] https://bmus.latticegroup.com/docs/bmus_4e_g7b.1.3.png
[12] https://bmus.latticegroup.com/file/bmus4eg7b14png
[13] https://bmus.latticegroup.com/docs/bmus_4e_g7b.1.4.png
[14] https://bmus.latticegroup.com/file/bmus4eg7b15png
[15] https://bmus.latticegroup.com/docs/bmus_4e_g7b.1.5.png
[16] https://bmus.latticegroup.com/docs/bmus_4e_t7b.2.pdf
[17] https://bmus.latticegroup.com/docs/bmus_4e_t7b.2.csv
[18] https://bmus.latticegroup.com/file/bmus4eg7b21png
[19] https://bmus.latticegroup.com/docs/bmus_4e_g7b.2.1.png
[20] https://bmus.latticegroup.com/file/bmus4eg7b22png
[21] https://bmus.latticegroup.com/docs/bmus_4e_g7b.2.2.png
[22] https://bmus.latticegroup.com/file/bmus4eg7b23png
[23] https://bmus.latticegroup.com/docs/bmus_4e_g7b.2.3.png
[24] https://bmus.latticegroup.com/file/bmus4eg7b24png
[25] https://bmus.latticegroup.com/docs/bmus_4e_g7b.2.4.png
[26] https://bmus.latticegroup.com/file/bmus4eg7b25png
[27] https://bmus.latticegroup.com/docs/bmus_4e_g7b.2.5.png
[28] https://bmus.latticegroup.com/docs/bmus_4e_t7b.3.pdf
[29] https://bmus.latticegroup.com/docs/bmus_4e_t7b.3.csv
[30] https://bmus.latticegroup.com/file/bmus4eg7b31png
[31] https://bmus.latticegroup.com/docs/bmus_4e_g7b.3.1.png
[32] https://bmus.latticegroup.com/docs/bmus_4e_t7b.4.1.pdf
[33] https://bmus.latticegroup.com/docs/bmus_4e_t7b.4.1.csv
[34] https://bmus.latticegroup.com/file/bmus4eg7b41png
[35] https://bmus.latticegroup.com/docs/bmus_4e_g7b.4.1.png
[36] https://bmus.latticegroup.com/file/bmus4eg7b42png
[37] https://bmus.latticegroup.com/docs/bmus_4e_g7b.4.2.png
[38] https://bmus.latticegroup.com/file/bmus4eg7b43png
[39] https://bmus.latticegroup.com/docs/bmus_4e_g7b.4.3.png
[40] https://bmus.latticegroup.com/file/bmus4eg7b44png
[41] https://bmus.latticegroup.com/docs/bmus_4e_g7b.4.4.png
[42] https://bmus.latticegroup.com/docs/bmus_4e_t7b.4.2.pdf
[43] https://bmus.latticegroup.com/docs/bmus_4e_t7b.4.2.csv
[44] https://bmus.latticegroup.com/file/bmus4eg7b45png
[45] https://bmus.latticegroup.com/docs/bmus_4e_g7b.4.5.png
[46] https://bmus.latticegroup.com/docs/bmus_4e_t7b.4.3.pdf
[47] https://bmus.latticegroup.com/docs/bmus_4e_t7b.4.3.csv
[48] https://bmus.latticegroup.com/docs/bmus_4e_t7b.4.4.pdf
[49] https://bmus.latticegroup.com/docs/bmus_4e_t7b.4.4.csv
[50] https://bmus.latticegroup.com/file/bmus4eg7b46png
[51] https://bmus.latticegroup.com/docs/bmus_4e_g7b.4.6.png
[52] https://bmus.latticegroup.com/docs/bmus_4e_t7b.5.1.pdf
[53] https://bmus.latticegroup.com/docs/bmus_4e_t7b.5.1.csv
[54] https://bmus.latticegroup.com/file/bmus4eg7b51png
[55] https://bmus.latticegroup.com/docs/bmus_4e_g7b.5.1.png
[56] https://bmus.latticegroup.com/file/bmus4eg7b52png
[57] https://bmus.latticegroup.com/docs/bmus_4e_g7b.5.2.png
[58] https://bmus.latticegroup.com/file/bmus4eg7b53png
[59] https://bmus.latticegroup.com/docs/bmus_4e_g7b.5.3.png
[60] http://www.who.int/chp/topics/Osteoporosis.pdf
[61] http://www.agingsociety.org/agingsociety/publications/chronic/index.html
[62] http://www.cdc.gov/HomeandRecreationalSafety/Falls/adulthipfx.html
[63] https://bmus.latticegroup.com/docs/bmus_4e_t7b.6.pdf
[64] https://bmus.latticegroup.com/docs/bmus_4e_t7b.6.csv
[65] https://bmus.latticegroup.com/file/bmus4eg7b6png
[66] https://bmus.latticegroup.com/docs/bmus_4e_g7b.6.png
[67] https://bmus.latticegroup.com/docs/bmus_4e_t7b.7.pdf
[68] https://bmus.latticegroup.com/docs/bmus_4e_t7b.7.csv
[69] https://bmus.latticegroup.com/file/bmus4eg7b7png
[70] https://bmus.latticegroup.com/docs/bmus_4e_g7b.7.png
[71] https://www.ownthebone.org/
[72] https://www.ncoa.org/news/resources-for-reporters/get-the-facts/falls-prevention-facts/
[73] https://www.cdc.gov/nchs/nhis/index.htm?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fnchs%2Fnhis.htm
[74] https://bmus.latticegroup.com/docs/bmus_4e_t7c.0.pdf
[75] https://bmus.latticegroup.com/docs/bmus_4e_t7c.0.csv
[76] https://bmus.latticegroup.com/docs/bmus_4e_t7c.0.1.pdf
[77] https://bmus.latticegroup.com/docs/bmus_4e_t7c.0.1.csv
[78] https://bmus.latticegroup.com/file/bmus4eg7c01png
[79] https://bmus.latticegroup.com/docs/bmus_4e_g7c.0.1.png
[80] https://bmus.latticegroup.com/docs/bmus_4e_t7c.0.2.pdf
[81] https://bmus.latticegroup.com/docs/bmus_4e_t7c.0.2.csv
[82] https://bmus.latticegroup.com/file/bmus4eg7c02png
[83] https://bmus.latticegroup.com/docs/bmus_4e_g7c.0.2.png
[84] https://www.boneandjointburden.org/fourth-edition/viic17/children-and-adolescent-icd-9-cm-codes
[85] https://www.hcup-us.ahrq.gov/
[86] https://bmus.latticegroup.com/file/bmus4et7c10jpg
[87] https://bmus.latticegroup.com/docs/bmus_4e_t7c.1.0.jpg
[88] https://www.cpsc.gov/cgibin/NEISSQuery/home.aspx
[89] http://report.nih.gov/fundingfacts/fundingfacts.aspx
[90] https://bmus.latticegroup.com/docs/bmus_4e_t7c.1.1.pdf
[91] https://bmus.latticegroup.com/docs/bmus_4e_t7c.1.1.csv
[92] https://bmus.latticegroup.com/file/bmus4eg7c11png
[93] https://bmus.latticegroup.com/docs/bmus_4e_g7c.1.1.png
[94] https://bmus.latticegroup.com/file/bmus4eg7c12png
[95] https://bmus.latticegroup.com/docs/bmus_4e_g7c.1.2.png
[96] https://bmus.latticegroup.com/docs/bmus_4e_t7c.1.2.pdf
[97] https://bmus.latticegroup.com/docs/bmus_4e_t7c.1.2.csv
[98] https://bmus.latticegroup.com/file/bmus4eg7c13png
[99] https://bmus.latticegroup.com/docs/bmus_4e_g7c.1.3.png
[100] https://bmus.latticegroup.com/file/bmus4eg7c14png
[101] https://bmus.latticegroup.com/docs/bmus_4e_g7c.1.4.png
[102] https://bmus.latticegroup.com/docs/bmus_4e_t7c.2.pdf
[103] https://bmus.latticegroup.com/docs/bmus_4e_t7c.2.csv
[104] https://bmus.latticegroup.com/file/bmus4eg7c21png
[105] https://bmus.latticegroup.com/docs/bmus_4e_g7c.2.1.png
[106] https://bmus.latticegroup.com/file/bmus4eg7c22png
[107] https://bmus.latticegroup.com/docs/bmus_4e_g7c.2.2.png
[108] https://bmus.latticegroup.com/docs/bmus_4e_t7c.3.pdf
[109] https://bmus.latticegroup.com/docs/bmus_4e_t7c.3.csv
[110] https://bmus.latticegroup.com/file/bmus4eg7c33png
[111] https://bmus.latticegroup.com/docs/bmus_4e_g7c.3.3.png
[112] https://bmus.latticegroup.com/file/bmus4eg7c31png
[113] https://bmus.latticegroup.com/docs/bmus_4e_g7c.3.1.png
[114] https://bmus.latticegroup.com/file/bmus4eg7c32png
[115] https://bmus.latticegroup.com/docs/bmus_4e_g7c.3.2.png
[116] https://www.aafp.org/afp/2014/1215/p843.html
[117] https://bmus.latticegroup.com/docs/bmus_4e_t7c.4.pdf
[118] https://bmus.latticegroup.com/docs/bmus_4e_t7c.4.csv
[119] https://bmus.latticegroup.com/file/bmus4eg7c41png
[120] https://bmus.latticegroup.com/docs/bmus_4e_g7c.4.1.png
[121] https://bmus.latticegroup.com/file/bmus4eg7c430png
[122] https://bmus.latticegroup.com/docs/bmus_4e_g7c.4.3.0.png
[123] https://bmus.latticegroup.com/file/bmus4eg7c42png
[124] https://bmus.latticegroup.com/docs/bmus_4e_g7c.4.2.png
[125] https://bmus.latticegroup.com/file/bmus4eg7c51png
[126] https://bmus.latticegroup.com/docs/bmus_4e_g7c.5.1.png
[127] https://bmus.latticegroup.com/docs/bmus_4e_t7c.1.5.pdf
[128] https://bmus.latticegroup.com/docs/bmus_4e_t7c.1.5.csv
[129] https://bmus.latticegroup.com/file/bmus4eg7c53png
[130] https://bmus.latticegroup.com/docs/bmus_4e_g7c.5.3.png
[131] https://bmus.latticegroup.com/file/bmus4eg7c52png
[132] https://bmus.latticegroup.com/docs/bmus_4e_g7c.5.2.png
[133] https://bmus.latticegroup.com/file/bmus4eg7c61png
[134] https://bmus.latticegroup.com/docs/bmus_4e_g7c.6.1.png
[135] https://bmus.latticegroup.com/docs/bmus_4e_t7c.6.pdf
[136] https://bmus.latticegroup.com/docs/bmus_4e_t7c.6.csv
[137] https://bmus.latticegroup.com/file/bmus4eg7c62png
[138] https://bmus.latticegroup.com/docs/bmus_4e_g7c.6.2.png
[139] https://bmus.latticegroup.com/file/bmus4eg7c71png
[140] https://bmus.latticegroup.com/docs/bmus_4e_g7c.7.1.png
[141] https://bmus.latticegroup.com/docs/bmus_4e_t7c.7.1.pdf
[142] https://bmus.latticegroup.com/docs/bmus_4e_t7c.7.1.csv
[143] https://bmus.latticegroup.com/file/bmus4eg7c72png
[144] https://bmus.latticegroup.com/docs/bmus_4e_g7c.7.2.png
[145] https://bmus.latticegroup.com/docs/bmus_4e_t7c.7.2.pdf
[146] https://bmus.latticegroup.com/docs/bmus_4e_t7c.7.2.csv
[147] https://bmus.latticegroup.com/file/bmus4eg7c73png
[148] https://bmus.latticegroup.com/docs/bmus_4e_g7c.7.3.png
[149] https://bmus.latticegroup.com/file/bmus4eg7c74png
[150] https://bmus.latticegroup.com/docs/bmus_4e_g7c.7.4.png
[151] https://bmus.latticegroup.com/file/bmus4eg7c75png
[152] https://bmus.latticegroup.com/docs/bmus_4e_g7c.7.5.png
[153] http://www.ncys.org/publications/2008-sports-participation-study.php
[154] http://www.physicalactivitycouncil.com/pdfs/current.pdf
[155] https://assets.aspeninstitute.org/content/uploads/2018/10/StateofPlay2018_v4WEB_2-FINAL.pdf
[156] https://www.boneandjointburden.org/fourth-edition/via0/tumors
[157] https://bmus.latticegroup.com/file/bmus4eg7c81png
[158] https://bmus.latticegroup.com/docs/bmus_4e_g7c.8.1.png
[159] https://bmus.latticegroup.com/docs/bmus_4e_t7c.8.pdf
[160] https://bmus.latticegroup.com/docs/bmus_4e_t7c.8.csv
[161] https://bmus.latticegroup.com/file/bmus4eg7c83png
[162] https://bmus.latticegroup.com/docs/bmus_4e_g7c.8.3.png
[163] https://bmus.latticegroup.com/file/bmus4eg7c82png
[164] https://bmus.latticegroup.com/docs/bmus_4e_g7c.8.2.png
[165] https://bmus.latticegroup.com/docs/bmus_4e_t7c.9.pdf
[166] https://bmus.latticegroup.com/docs/bmus_4e_t7c.9.csv
[167] https://bmus.latticegroup.com/file/bmus4eg7c91png
[168] https://bmus.latticegroup.com/docs/bmus_4e_g7c.9.1.png
[169] https://bmus.latticegroup.com/file/bmus4eg7c92png
[170] https://bmus.latticegroup.com/docs/bmus_4e_g7c.9.2.png
[171] https://bmus.latticegroup.com/docs/bmus_4e_t7c.10.pdf
[172] https://bmus.latticegroup.com/docs/bmus_4e_t7c.10.csv
[173] https://bmus.latticegroup.com/file/bmus4eg7c101png
[174] https://bmus.latticegroup.com/docs/bmus_4e_g7c.10.1.png
[175] https://bmus.latticegroup.com/file/bmus4eg7c102png
[176] https://bmus.latticegroup.com/docs/bmus_4e_g7c.10.2.png
[177] http://www.cdc.gov/arthritis/basics/childhood.htm
[178] https://bmus.latticegroup.com/file/bmus4eg7c111png
[179] https://bmus.latticegroup.com/docs/bmus_4e_g7c.11.1.png
[180] https://bmus.latticegroup.com/docs/bmus_4e_t7c.11.pdf
[181] https://bmus.latticegroup.com/docs/bmus_4e_t7c.11.csv
[182] https://bmus.latticegroup.com/file/bmus4eg7c112png
[183] https://bmus.latticegroup.com/docs/bmus_4e_g7c.11.2.png
[184] https://bmus.latticegroup.com/file/bmus4eg7c113png
[185] https://bmus.latticegroup.com/docs/bmus_4e_g7c.11.3.png
[186] https://stopchildhoodpain.org/
[187] https://bmus.latticegroup.com/file/bmus4eg7c121png
[188] https://bmus.latticegroup.com/docs/bmus_4e_g7c.12.1.png
[189] https://bmus.latticegroup.com/docs/bmus_4e_t7c.12.pdf
[190] https://bmus.latticegroup.com/docs/bmus_4e_t7c.12.csv
[191] https://bmus.latticegroup.com/file/bmus4eg7c122png
[192] https://bmus.latticegroup.com/docs/bmus_4e_g7c.12.2.png
[193] https://www.boneandjointburden.org/fourth-edition/iiib70/joint-pain-and-joint-replacement
[194] http://dx.doi.org/10.1007/s11999-016-4903-3
[195] https://bmus.latticegroup.com/docs/bmus_4e_t7c.13.pdf
[196] https://bmus.latticegroup.com/docs/bmus_4e_t7c.13.csv
[197] https://bmus.latticegroup.com/file/bmus4eg7c141png
[198] https://bmus.latticegroup.com/docs/bmus_4e_g7c.14.1.png
[199] https://bmus.latticegroup.com/file/bmus4eg7c142png
[200] https://bmus.latticegroup.com/docs/bmus_4e_g7c.14.2.png
[201] https://bmus.latticegroup.com/file/bmus4eg7c143png
[202] https://bmus.latticegroup.com/docs/bmus_4e_g7c.14.3.png
[203] https://bmus.latticegroup.com/docs/bmus_e4_t7d.1.pdf
[204] https://bmus.latticegroup.com/docs/bmus_e4_t7d.1.csv
[205] https://bmus.latticegroup.com/file/bmuse4g7d01png
[206] https://bmus.latticegroup.com/docs/bmus_e4_g7d.0.1.png
[207] https://bmus.latticegroup.com/file/bmuse4g7d02png
[208] https://bmus.latticegroup.com/docs/bmus_e4_g7d.0.2.png
[209] https://bmus.latticegroup.com/file/bmuse4g7d03png
[210] https://bmus.latticegroup.com/docs/bmus_e4_g7d.0.3.png
[211] https://bmus.latticegroup.com/file/bmuse4g7d04png
[212] https://bmus.latticegroup.com/docs/bmus_e4_g7d.0.4.png
[213] https://bmus.latticegroup.com/docs/bmus_e4_t7d.2.pdf
[214] https://bmus.latticegroup.com/docs/bmus_e4_t7d.2.csv
[215] https://bmus.latticegroup.com/file/bmuse4g7d11png
[216] https://bmus.latticegroup.com/docs/bmus_e4_g7d.1.1.png
[217] https://bmus.latticegroup.com/file/bmuse4g7d12png
[218] https://bmus.latticegroup.com/docs/bmus_e4_g7d.1.2.png
[219] https://bmus.latticegroup.com/file/bmuse4g7d13png
[220] https://bmus.latticegroup.com/docs/bmus_e4_g7d.1.3.png
[221] https://bmus.latticegroup.com/file/bmuse4g7d14png
[222] https://bmus.latticegroup.com/docs/bmus_e4_g7d.1.4.png
[223] https://bmus.latticegroup.com/file/bmuse4g7d20png
[224] https://bmus.latticegroup.com/docs/bmus_e4_g7d.2.0.png
[225] https://bmus.latticegroup.com/docs/bmus_e4_t7d.4.1.pdf
[226] https://bmus.latticegroup.com/docs/bmus_e4_t7d.4.1.csv
[227] https://bmus.latticegroup.com/file/bmuse4g7d21png
[228] https://bmus.latticegroup.com/docs/bmus_e4_g7d.2.1.png
[229] https://bmus.latticegroup.com/file/bmuse4g7d221png
[230] https://bmus.latticegroup.com/docs/bmus_e4_g7d.2.2.1.png
[231] https://bmus.latticegroup.com/file/bmuse4g7d222png
[232] https://bmus.latticegroup.com/docs/bmus_e4_g7d.2.2.2.png
[233] https://bmus.latticegroup.com/docs/bmus_e4_t7d.4.2.pdf
[234] https://bmus.latticegroup.com/docs/bmus_e4_t7d.4.2.csv
[235] https://bmus.latticegroup.com/file/bmuse4g7d25png
[236] https://bmus.latticegroup.com/docs/bmus_e4_g7d.2.5.png
[237] https://bmus.latticegroup.com/file/bmuse4g7d23png
[238] https://bmus.latticegroup.com/docs/bmus_e4_g7d.2.3.png
[239] https://bmus.latticegroup.com/file/bmuse4g7d24png
[240] https://bmus.latticegroup.com/docs/bmus_e4_g7d.2.4.png
[241] https://bmus.latticegroup.com/docs/bmus_e4_t7d.4.3.pdf
[242] https://bmus.latticegroup.com/docs/bmus_e4_t7d.4.3.csv
[243] https://bmus.latticegroup.com/file/bmuse4g7d26png
[244] https://bmus.latticegroup.com/docs/bmus_e4_g7d.2.6.png
[245] https://www.cdc.gov/mmwr/preview/mmwrhtml/00041424.htm
[246] http://www.cdc.gov/pcd/issues/2010/may/10_0035.htm
[247] http://healthfinder. gov/news/newsstorv.aspx?Docid=658088
[248] https://bmus.latticegroup.com/file/bmuse4g7d31png
[249] https://bmus.latticegroup.com/docs/bmus_e4_g7d.3.1.png
[250] https://bmus.latticegroup.com/docs/bmus_e4_t7d.5.pdf
[251] https://bmus.latticegroup.com/docs/bmus_e4_t7d.5.csv
[252] https://bmus.latticegroup.com/file/bmuse4g7d32png
[253] https://bmus.latticegroup.com/docs/bmus_e4_g7d.3.2.png
[254] https://bmus.latticegroup.com/docs/bmus_e4_t7d.6.pdf
[255] https://bmus.latticegroup.com/docs/bmus_e4_t7d.6.csv
[256] https://bmus.latticegroup.com/file/bmuse4g7d41png
[257] https://bmus.latticegroup.com/docs/bmus_e4_g7d.4.1.png
[258] https://bmus.latticegroup.com/file/bmuse4g7d42png
[259] https://bmus.latticegroup.com/docs/bmus_e4_g7d.4.2.png
[260] https://bmus.latticegroup.com/file/bmuse4g7d43png
[261] https://bmus.latticegroup.com/docs/bmus_e4_g7d.4.3.png
[262] https://bmus.latticegroup.com/docs/bmus_e4_t7d.7.pdf
[263] https://bmus.latticegroup.com/docs/bmus_e4_t7d.7.csv
[264] https://bmus.latticegroup.com/file/bmuse4g7d51png
[265] https://bmus.latticegroup.com/docs/bmus_e4_g7d.5.1.png
[266] https://bmus.latticegroup.com/file/bmuse4g7d52png
[267] https://bmus.latticegroup.com/docs/bmus_e4_g7d.5.2.png